Polycrystalline materials consist of numerous small crystals or grains, which influence properties such as strength, conductivity, and resistance to corrosion. Their unique grain boundaries impact mechanical behavior and electrical performance, making them ideal for applications like solar panels and electronic devices. Discover how polycrystalline structures can enhance your projects by exploring the detailed insights in this article.
Table of Comparison
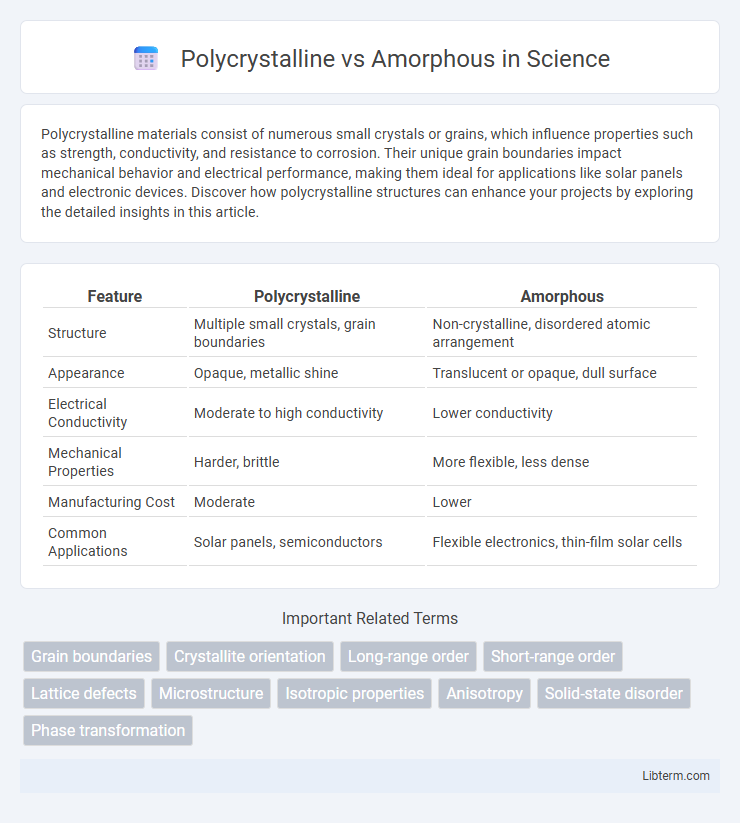
| Feature | Polycrystalline | Amorphous |
|---|---|---|
| Structure | Multiple small crystals, grain boundaries | Non-crystalline, disordered atomic arrangement |
| Appearance | Opaque, metallic shine | Translucent or opaque, dull surface |
| Electrical Conductivity | Moderate to high conductivity | Lower conductivity |
| Mechanical Properties | Harder, brittle | More flexible, less dense |
| Manufacturing Cost | Moderate | Lower |
| Common Applications | Solar panels, semiconductors | Flexible electronics, thin-film solar cells |
Introduction to Polycrystalline and Amorphous Materials
Polycrystalline materials consist of numerous small crystals or grains, each with a distinct orientation, resulting in a structure that influences mechanical and electrical properties. Amorphous materials lack a long-range ordered crystal structure, leading to isotropic behavior and unique optical and electrical characteristics. Understanding the differences between polycrystalline and amorphous materials is crucial for applications in electronics, solar cells, and structural components.
Defining Polycrystalline Structures
Polycrystalline structures consist of numerous small crystals or grains, each with distinct orientations, forming a solid material without a single continuous lattice. These grains are separated by grain boundaries that influence mechanical, electrical, and thermal properties. Unlike amorphous materials, which lack long-range order, polycrystalline solids exhibit crystalline order within each grain, offering a balance between structural rigidity and defect tolerance.
Understanding Amorphous Materials
Amorphous materials lack the long-range order characteristic of polycrystalline structures, resulting in unique physical properties such as isotropic behavior and enhanced flexibility. Their atomic arrangement is random, which influences electrical conductivity and optical transparency in applications like thin-film solar cells and flexible electronics. Understanding the disordered atomic network allows for tailoring amorphous materials to improve performance in semiconductor devices and anti-reflective coatings.
Formation Processes and Manufacturing
Polycrystalline materials form through the controlled solidification of molten substances, resulting in numerous small crystals or grains that influence mechanical and electrical properties. In contrast, amorphous materials are produced by rapid cooling or quenching, preventing the formation of a regular crystal lattice and yielding a non-crystalline structure. Manufacturing polycrystalline components often involves annealing and sintering techniques, while amorphous materials are commonly manufactured using rapid solidification processes such as melt spinning or vapor deposition.
Structural Differences: Grains vs. Disordered Atoms
Polycrystalline materials consist of numerous small grains or crystallites, each with a well-ordered atomic arrangement separated by grain boundaries. Amorphous materials lack this long-range order, featuring atoms arranged randomly without a defined crystalline structure. The presence of grains in polycrystalline materials impacts mechanical strength and electrical conductivity, whereas the disordered atomic structure in amorphous materials often results in distinct optical and thermal properties.
Mechanical and Physical Properties Comparison
Polycrystalline materials exhibit higher mechanical strength and better thermal conductivity due to their orderly grain structure, whereas amorphous materials lack a long-range order, resulting in lower strength but superior flexibility and impact resistance. Polycrystalline solids tend to have distinct melting points and greater hardness, while amorphous solids soften over a temperature range and have lower density. The differences in atomic arrangement cause polycrystalline materials to be more brittle, contrasting with the ductile nature of amorphous substances.
Performance in Solar Cell Applications
Polycrystalline solar cells exhibit higher efficiency and better charge carrier mobility compared to amorphous solar cells, making them more suitable for high-performance applications. Amorphous solar cells, characterized by disordered atomic structures, typically have lower energy conversion efficiency but offer advantages in flexibility and cost-effectiveness. The choice between polycrystalline and amorphous materials impacts solar cell performance, longevity, and suitability for different installation environments.
Cost and Availability Considerations
Polycrystalline solar panels generally offer a lower cost per watt due to simpler manufacturing processes and widespread availability in the market, making them a cost-effective choice for many solar projects. Amorphous solar panels, while typically more expensive to produce because of specialized thin-film technology, provide flexibility and perform better in low-light conditions but remain less commonly available. The balance between upfront cost and performance benefits influences the selection depending on project budget and environmental factors.
Environmental Impact and Sustainability
Polycrystalline solar panels generally offer higher energy efficiency and longer lifespan, resulting in greater environmental benefits through reduced carbon emissions over their operational period. Amorphous solar panels consume fewer raw materials and energy during production, leading to a smaller initial environmental footprint and enhanced sustainability in resource usage. Both technologies contribute to renewable energy goals, but polycrystalline panels excel in long-term energy yield while amorphous panels provide advantages in material efficiency and manufacturing sustainability.
Choosing Between Polycrystalline and Amorphous Materials
Choosing between polycrystalline and amorphous materials depends on the specific application requirements, such as mechanical strength, electrical conductivity, and cost effectiveness. Polycrystalline materials offer higher strength and better conductivity due to their ordered grain structure, making them ideal for structural and electronic applications. Amorphous materials lack long-range order, providing superior flexibility and corrosion resistance, which is advantageous in coatings and specialized glass manufacturing.
Polycrystalline Infographic
 libterm.com
libterm.com