Antibody receptors are specialized proteins on immune cells that bind to antibodies, facilitating the recognition and elimination of pathogens. These receptors play a crucial role in immune response by triggering cellular activities such as phagocytosis and antibody-dependent cellular cytotoxicity. Explore the rest of the article to understand how antibody receptors contribute to immunity and impact your health.
Table of Comparison
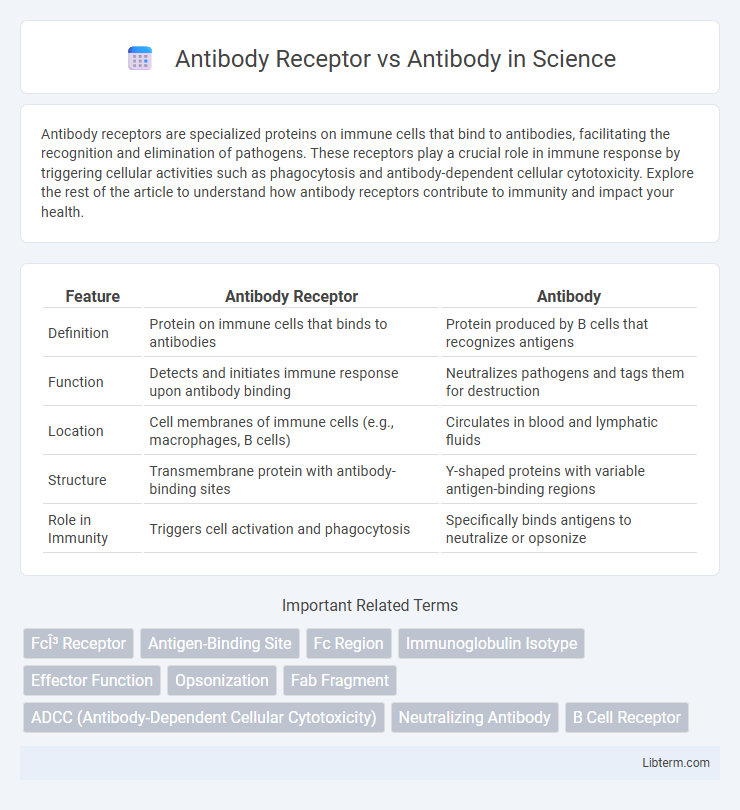
| Feature | Antibody Receptor | Antibody |
|---|---|---|
| Definition | Protein on immune cells that binds to antibodies | Protein produced by B cells that recognizes antigens |
| Function | Detects and initiates immune response upon antibody binding | Neutralizes pathogens and tags them for destruction |
| Location | Cell membranes of immune cells (e.g., macrophages, B cells) | Circulates in blood and lymphatic fluids |
| Structure | Transmembrane protein with antibody-binding sites | Y-shaped proteins with variable antigen-binding regions |
| Role in Immunity | Triggers cell activation and phagocytosis | Specifically binds antigens to neutralize or opsonize |
Overview of Antibodies and Antibody Receptors
Antibodies are Y-shaped proteins produced by B cells that specifically bind to antigens, facilitating immune response and pathogen neutralization. Antibody receptors, such as Fc receptors found on immune cells, recognize and bind the Fc region of antibodies, mediating processes like phagocytosis and antibody-dependent cellular cytotoxicity (ADCC). Understanding the interaction between antibodies and their receptors is critical for designing targeted immunotherapies and enhancing immune system effectiveness.
Structural Differences: Antibody vs Antibody Receptor
Antibody receptors, primarily found on immune cells, differ structurally from antibodies in that they possess transmembrane domains enabling cell surface integration, whereas antibodies are soluble proteins with variable and constant regions forming Y-shaped structures. Unlike antibodies that directly bind antigens through their Fab regions, antibody receptors recognize the Fc region of antibodies to mediate immune responses. The structural divergence facilitates antibody receptors' role in signal transduction and cellular activation, contrasting with antibodies' antigen recognition and neutralization functions.
Functional Roles in the Immune System
Antibody receptors, primarily expressed on immune cells like B cells and macrophages, bind antibodies to mediate immune responses such as phagocytosis and antibody-dependent cellular cytotoxicity (ADCC). Antibodies, produced by plasma cells, specifically recognize and neutralize pathogens, tagging them for destruction or preventing viral entry into host cells. Together, antibody receptors facilitate communication between antibodies and immune cells, enhancing pathogen clearance and immune regulation.
Mechanisms of Antigen Recognition
Antibody receptors, such as B cell receptors (BCRs), recognize antigens by binding specific epitopes with membrane-bound immunoglobulins, initiating intracellular signaling cascades for immune activation. Free antibodies, secreted by plasma cells, identify antigens through their variable regions, neutralizing pathogens or marking them for immune clearance via opsonization or complement activation. The key difference lies in antibody receptors' role in antigen detection and cellular activation, whereas antibodies mediate direct pathogen neutralization and effector functions.
Types and Classes of Antibodies
Antibody receptors, such as Fc receptors, specifically bind to the constant region of antibodies, enabling immune cells to recognize and respond to pathogens. Antibodies are classified into five main classes--IgG, IgA, IgM, IgE, and IgD--each with distinct functions and structural variations that influence their interaction with different antibody receptors. The specificity of antibody-receptor binding is critical for processes like phagocytosis, antibody-dependent cellular cytotoxicity, and immune regulation.
Types and Families of Antibody Receptors
Antibody receptors, primarily classified into Fc receptors (FcRs), include families such as FcgRs, FceRs, and FcaRs, each specific to different antibody isotypes like IgG, IgE, and IgA. These receptors vary in their cellular distribution and function, mediating immune responses like phagocytosis, antibody-dependent cellular cytotoxicity (ADCC), and allergic reactions. Antibodies themselves belong to distinct classes--IgG, IgA, IgM, IgE, and IgD--each with unique roles in adaptive immunity, while their corresponding receptors precisely regulate downstream effector mechanisms.
Signaling Pathways: Antibody vs Receptor Activation
Antibody receptor activation triggers specific intracellular signaling pathways, such as the phosphorylation of ITAM (Immunoreceptor Tyrosine-based Activation Motif) motifs, leading to immune cell activation and cytokine release. In contrast, antibodies themselves do not initiate signaling but function by binding antigens to neutralize pathogens or mark them for clearance. Understanding the distinct roles of antibody binding versus receptor-mediated signaling is crucial for designing targeted immunotherapies and vaccines.
Clinical Implications and Therapeutic Uses
Antibody receptors, such as Fc receptors on immune cells, mediate crucial clinical functions by enabling antibody-dependent cellular cytotoxicity and phagocytosis, impacting treatment efficacy in immunotherapy. Therapeutic antibodies are designed to specifically bind antigens and recruit these receptors to target and eliminate diseased cells, including cancers and infections. Understanding the interaction between antibodies and their receptors optimizes drug design and enhances clinical outcomes in autoimmune diseases, cancer immunotherapy, and infectious disease management.
Key Research Advances and Discoveries
Recent key research advances have elucidated the distinct roles of antibody receptors, such as Fc receptors, in mediating immune responses by binding to the Fc region of antibodies and facilitating processes like antibody-dependent cellular cytotoxicity (ADCC) and phagocytosis. Discoveries in antibody engineering have enhanced therapeutic antibody design by optimizing Fc receptor interactions to improve efficacy and reduce adverse effects. Advanced structural studies have revealed the molecular mechanisms of antibody-receptor binding specificity, enabling the development of targeted immunotherapies and novel diagnostic tools.
Future Directions in Antibody and Receptor Research
Future directions in antibody and antibody receptor research emphasize enhancing specificity and affinity through advanced protein engineering and high-throughput screening technologies. Emerging techniques like CRISPR-based receptor editing and single-cell sequencing enable precise modulation of antibody-receptor interactions, facilitating novel therapeutic strategies. Integrating artificial intelligence and machine learning accelerates the discovery of antibody variants optimized for targeting complex diseases and improving immunotherapy efficacy.
Antibody Receptor Infographic
 libterm.com
libterm.com