Catholyte is an electrolyzed solution used as a powerful disinfectant and cleaning agent with applications in medical, agricultural, and industrial settings. It contains reactive oxygen species that effectively eliminate bacteria, viruses, and fungi while being safe for surfaces and skin. Discover how catholyte can enhance your hygiene practices by exploring the rest of this article.
Table of Comparison
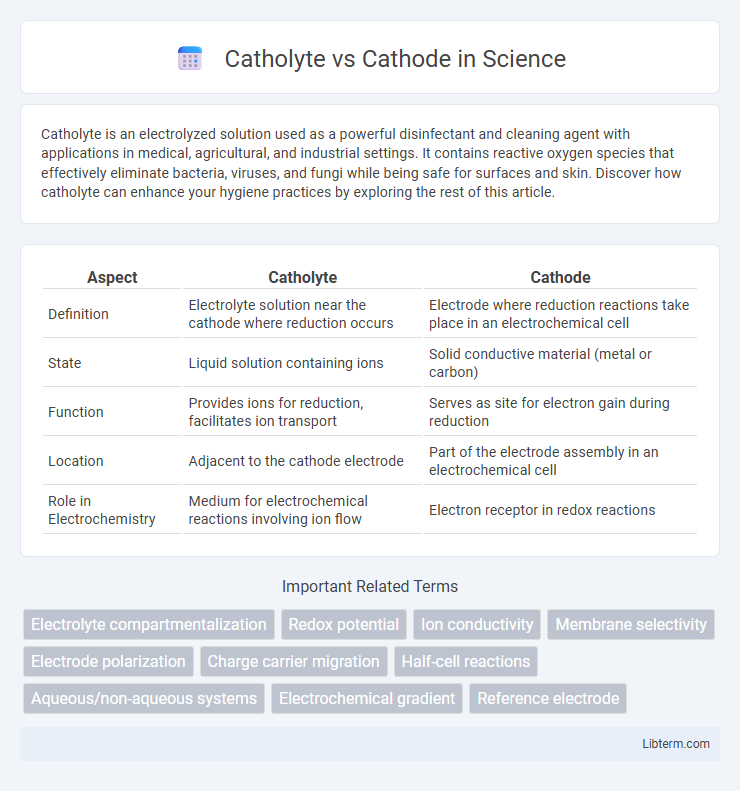
| Aspect | Catholyte | Cathode |
|---|---|---|
| Definition | Electrolyte solution near the cathode where reduction occurs | Electrode where reduction reactions take place in an electrochemical cell |
| State | Liquid solution containing ions | Solid conductive material (metal or carbon) |
| Function | Provides ions for reduction, facilitates ion transport | Serves as site for electron gain during reduction |
| Location | Adjacent to the cathode electrode | Part of the electrode assembly in an electrochemical cell |
| Role in Electrochemistry | Medium for electrochemical reactions involving ion flow | Electron receptor in redox reactions |
Introduction to Catholyte and Cathode
Catholyte is an electrolyte solution that facilitates the flow of ions toward the cathode in electrochemical cells, playing a crucial role in energy storage systems like flow batteries. The cathode is the electrode where the reduction reaction occurs, accepting electrons and interacting directly with the catholyte during electrochemical processes. Understanding the distinct functions of catholyte and cathode is essential for optimizing battery performance and efficiency.
Defining Catholyte: Role and Function
Catholyte refers to the electrolyte solution in the cathode compartment of an electrochemical cell, facilitating ion transport and maintaining charge balance during redox reactions. Its primary function is to provide a medium for the reduction reaction at the cathode, enabling efficient electron transfer and sustaining the cell's electrical output. Unlike the cathode, which is the electrode where reduction occurs, the catholyte is the surrounding conductive fluid essential for electrochemical stability and performance.
Understanding the Cathode in Electrochemical Systems
The cathode in electrochemical systems serves as the electrode where reduction reactions occur, attracting cations from the electrolyte. Catholyte refers to the liquid electrolyte adjacent to the cathode, containing dissolved ions that participate in these reduction processes. Understanding the cathode's role involves analyzing its material composition and surface properties to optimize ion transfer and overall cell efficiency.
Key Differences Between Catholyte and Cathode
Catholyte refers to the electrolyte solution in an electrochemical cell where reduction occurs, containing ions that participate in the reaction, while the cathode is the electrode at which the reduction reaction takes place. The catholyte's main function is to transport ions to the cathode, facilitating charge transfer and maintaining the cell's electrical neutrality. Key differences include the physical state--catholyte being a liquid medium and cathode a solid conductive material--and their distinct roles in charge conduction and electrochemical reactions.
Chemical Composition: Catholyte vs Cathode
Catholyte typically consists of an electrolyte solution containing positively charged ions (cations) that facilitate ion transport in electrochemical cells, often comprising aqueous solutions of salts, acids, or bases. In contrast, the cathode is a solid electrode material, usually composed of metals or metal oxides, that serves as the site for reduction reactions in batteries or fuel cells. The chemical composition of the catholyte influences the ion conductivity and electrochemical stability, while the cathode's material determines the electrode potential and reaction kinetics.
Applications in Batteries and Fuel Cells
Catholyte refers to the electrolyte solution in the cathode compartment of batteries and fuel cells, playing a crucial role in ion transport and enzyme activity enhancement in bioelectrochemical systems. The cathode is the electrode where reduction reactions occur, essential for electron flow and energy output in devices such as lithium-ion batteries and proton exchange membrane fuel cells (PEMFCs). Applications in flow batteries, including vanadium redox flow batteries, rely on optimized catholyte compositions to improve stability and capacity, while cathode material advancements focus on enhancing conductivity, catalytic activity, and overall energy efficiency.
Performance Impacts: How Each Affects Efficiency
Catholyte composition directly influences the electrochemical reactions within a battery, impacting ion conductivity and overall cell efficiency. The cathode material determines the battery's voltage, capacity, and cycle life by facilitating electron transfer and structural stability during charge-discharge cycles. Optimizing both catholyte formulation and cathode architecture is crucial to enhancing energy density and minimizing internal resistance, thereby maximizing efficiency in advanced energy storage systems.
Selection Criteria: When to Use Catholyte or Cathode
Choosing between catholyte and cathode depends on the electrochemical system design and application requirements. Catholyte is preferred in flow batteries and systems needing ion transport through liquid electrolytes for enhanced conductivity and ease of maintenance, whereas solid cathodes are ideal in standard batteries where high energy density and structural stability are critical. Selection criteria include conductivity, material compatibility, system complexity, and operational temperature range.
Advances in Catholyte and Cathode Materials
Recent advances in catholyte and cathode materials have significantly enhanced battery performance by improving energy density and cycle life. Innovative catholytes featuring high ionic conductivity and stability, such as solid-state electrolytes, have reduced dendrite formation and increased safety in lithium-metal batteries. Simultaneously, cathode materials like layered lithium nickel manganese cobalt oxides (NMC) and high-voltage spinels are engineered for higher capacity and thermal stability, accelerating the development of next-generation energy storage solutions.
Future Trends in Electrochemical Technology
Catholyte formulations are evolving with enhanced ion conductivity and stability, enabling higher efficiency in next-generation electrochemical cells compared to traditional cathode materials. Advanced cathodes are integrating nanoscale architectures and novel composites to boost energy density and cycle life in batteries and fuel cells. Innovations in catholyte and cathode design are driving sustainable energy storage solutions and solid-state technologies for future electrification demands.
Catholyte Infographic
 libterm.com
libterm.com