Racemic mixtures contain equal amounts of two enantiomers of a chiral molecule, resulting in no net optical activity. Understanding the properties and applications of racemic compounds is essential in fields like pharmaceuticals, where the efficacy and safety of drugs can depend on their chirality. Explore the rest of this article to discover how racemic mixtures impact your daily life and industry.
Table of Comparison
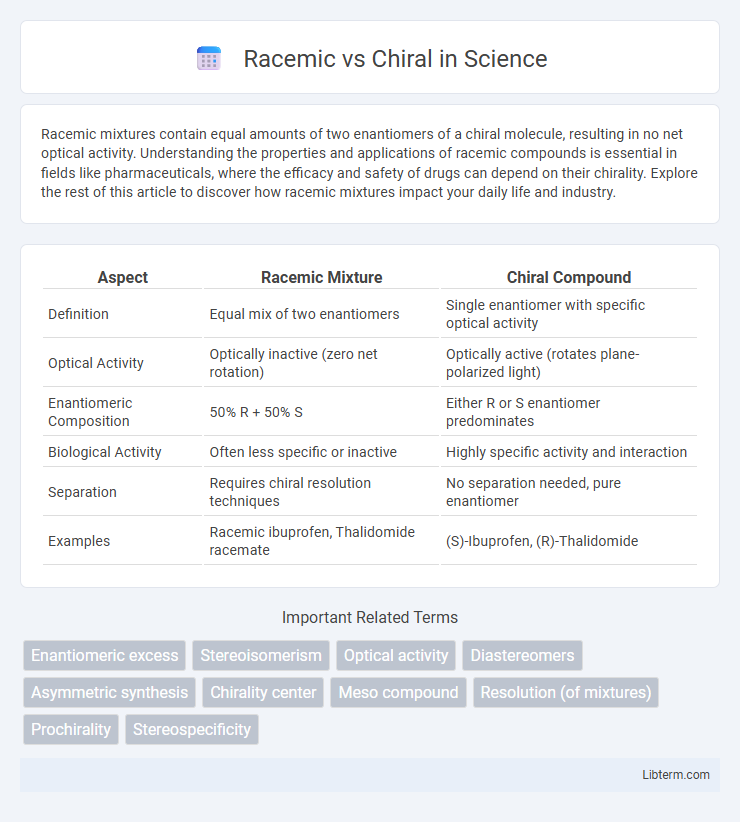
| Aspect | Racemic Mixture | Chiral Compound |
|---|---|---|
| Definition | Equal mix of two enantiomers | Single enantiomer with specific optical activity |
| Optical Activity | Optically inactive (zero net rotation) | Optically active (rotates plane-polarized light) |
| Enantiomeric Composition | 50% R + 50% S | Either R or S enantiomer predominates |
| Biological Activity | Often less specific or inactive | Highly specific activity and interaction |
| Separation | Requires chiral resolution techniques | No separation needed, pure enantiomer |
| Examples | Racemic ibuprofen, Thalidomide racemate | (S)-Ibuprofen, (R)-Thalidomide |
Introduction to Racemic and Chiral Compounds
Racemic compounds consist of equal amounts of left- and right-handed enantiomers, resulting in a non-optically active mixture. Chiral compounds contain molecules that are non-superimposable on their mirror images, leading to optical activity and distinct biological interactions. The study of racemic versus chiral compounds is crucial in pharmaceutical development due to differences in drug efficacy and metabolism.
Understanding Chirality: Definition and Importance
Chirality is a fundamental property of molecules where a structure cannot be superimposed on its mirror image, resulting in two enantiomers with distinct spatial arrangements. Racemic mixtures contain equal amounts of both enantiomers, often leading to different chemical and biological behaviors compared to pure chiral compounds. Understanding chirality is crucial in pharmaceuticals, as the therapeutic effects and safety profiles of drugs depend significantly on the specific enantiomer present.
Racemic Mixtures: Composition and Properties
Racemic mixtures consist of equal amounts of two enantiomers, each being a mirror image but non-superimposable chiral molecule. These mixtures exhibit no optical activity because the opposite rotations of plane-polarized light by each enantiomer cancel out. They often differ in physical properties from pure enantiomers, such as melting point and solubility, influencing their behavior in chemical and pharmaceutical applications.
Key Differences Between Racemic and Chiral Molecules
Racemic molecules contain equal amounts of left- and right-handed enantiomers, making them optically inactive, whereas chiral molecules consist of a single enantiomer that is optically active. The key difference lies in their optical activity; racemic mixtures do not rotate plane-polarized light, while chiral molecules do. This distinction impacts their chemical behavior and interactions, especially in pharmaceuticals where chiral specificity is crucial for drug efficacy and safety.
Stereochemistry in Drug Development
Racemic mixtures contain equal amounts of both enantiomers of a chiral molecule, often leading to variable pharmacological effects due to differences in stereochemistry. Chiral drugs consist of a single enantiomer, which can provide enhanced efficacy and reduced side effects by targeting specific biological receptors more precisely. Understanding stereochemistry is crucial in drug development to optimize therapeutic outcomes and minimize adverse reactions associated with enantiomeric forms.
Biological Significance of Chiral Compounds
Chiral compounds play a critical role in biological systems due to their ability to interact selectively with enzymes, receptors, and other biomolecules, which are often chiral themselves. Unlike racemic mixtures containing equal amounts of enantiomers, chiral compounds exhibit distinct pharmacological and biochemical activities, influencing drug efficacy and metabolism. Understanding chirality is essential for developing pharmaceuticals with targeted therapeutic effects and minimizing adverse side effects.
Methods for Separating Racemic Mixtures
Methods for separating racemic mixtures include chiral resolution techniques such as crystallization, chromatography, and enzymatic resolution. Chiral chromatography utilizes chiral stationary phases to differentiate enantiomers based on their interaction with the chiral environment, allowing effective separation. Enzymatic resolution employs stereospecific enzymes to selectively convert or degrade one enantiomer, facilitating isolation from the racemic mixture.
Industrial Applications of Chiral and Racemic Substances
Chiral substances in industrial applications are critical for producing enantiomerically pure pharmaceuticals, agrochemicals, and flavors, ensuring higher efficacy and reduced side effects. Racemic mixtures are often used when the separation of enantiomers is unnecessary or economically unfeasible, such as in certain solvents, antifreeze agents, and bulk chemical production. The choice between chiral and racemic compounds directly impacts product performance, regulatory approval, and cost-efficiency in large-scale manufacturing.
Analytical Techniques for Chiral Identification
Chiral identification relies heavily on analytical techniques such as chiral chromatography, including high-performance liquid chromatography (HPLC) with chiral stationary phases, enabling separation of enantiomers in racemic mixtures. Spectroscopic methods like circular dichroism (CD) and vibrational circular dichroism (VCD) provide stereochemical information by measuring differential absorption of circularly polarized light by chiral molecules. Nuclear magnetic resonance (NMR) spectroscopy combined with chiral shift reagents also facilitates differentiation between enantiomers by inducing chemical shift differences, critical for precise enantiomeric excess determination.
Future Trends in Chiral Chemistry
Future trends in chiral chemistry emphasize the development of more efficient asymmetric synthesis methods and advanced chiral catalysts to achieve higher enantioselectivity. Innovations in computational chemistry and machine learning are accelerating the design of novel chiral molecules with specific biological activities. Growing demand in pharmaceuticals and agrochemicals drives research towards sustainable and scalable chiral separation techniques, including supercritical fluid chromatography and membrane technology.
Racemic Infographic
 libterm.com
libterm.com