Substrate serves as the foundational layer in various fields such as biology, electronics, and materials science, providing essential support and interaction for overlying structures or processes. In biology, it refers to the substance an enzyme acts upon, while in electronics, it acts as the base material for circuits and components. Explore the rest of the article to discover how substrate's unique properties impact diverse applications and why understanding it can enhance your knowledge in these areas.
Table of Comparison
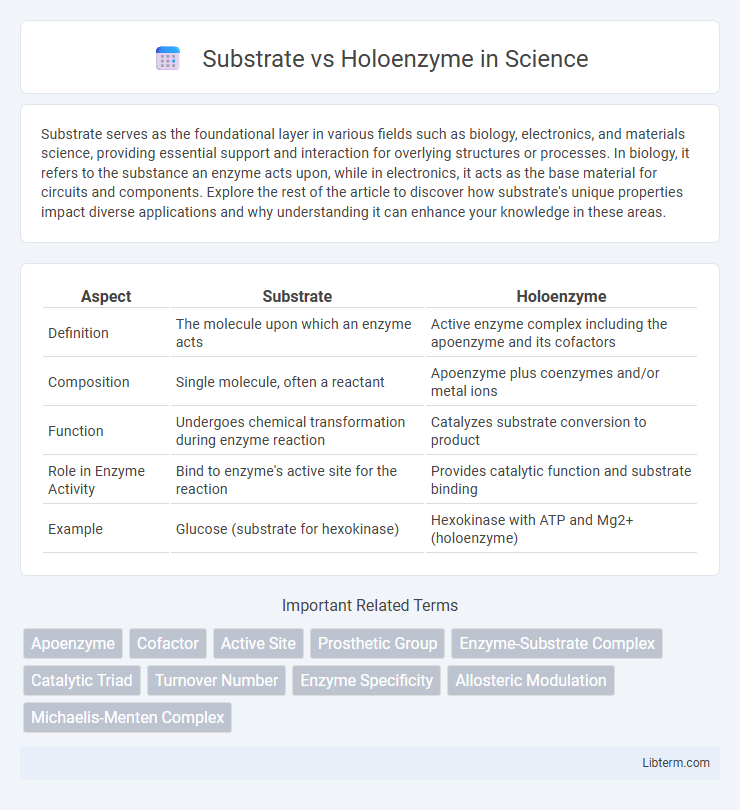
| Aspect | Substrate | Holoenzyme |
|---|---|---|
| Definition | The molecule upon which an enzyme acts | Active enzyme complex including the apoenzyme and its cofactors |
| Composition | Single molecule, often a reactant | Apoenzyme plus coenzymes and/or metal ions |
| Function | Undergoes chemical transformation during enzyme reaction | Catalyzes substrate conversion to product |
| Role in Enzyme Activity | Bind to enzyme's active site for the reaction | Provides catalytic function and substrate binding |
| Example | Glucose (substrate for hexokinase) | Hexokinase with ATP and Mg2+ (holoenzyme) |
Understanding the Basics: What Are Substrate and Holoenzyme?
A substrate is a specific molecule upon which an enzyme acts to catalyze a biochemical reaction, binding to the enzyme's active site to form an enzyme-substrate complex. A holoenzyme refers to the complete, active form of an enzyme, consisting of the apoenzyme (protein component) combined with its necessary cofactor or coenzyme, essential for catalytic activity. Understanding the distinction between substrate and holoenzyme is critical for grasping enzyme functionality and reaction mechanisms in biochemistry.
Structural Differences Between Substrate and Holoenzyme
Substrates are specific molecules that bind to an enzyme's active site, undergoing chemical transformation during the reaction process, while holoenzymes are complete enzymatic complexes composed of the apoenzyme (protein portion) and necessary cofactors or coenzymes. Structurally, the substrate lacks enzyme components and is typically a small molecule, whereas the holoenzyme exhibits a multi-subunit architecture with distinct binding sites crucial for catalysis and regulation. The holoenzyme's conformational flexibility allows precise interaction with substrates, enhancing catalytic efficiency through induced fit mechanisms.
Functional Roles in Enzymatic Reactions
Substrates serve as the specific molecules upon which enzymes act, undergoing chemical transformation during enzymatic reactions. Holoenzymes consist of both the apoenzyme (protein portion) and its necessary cofactors or coenzymes, enabling catalytic activity. The substrate binds to the holoenzyme's active site, facilitating the conversion to product through precise molecular interactions.
Mechanism of Enzyme-Substrate Interaction
The mechanism of enzyme-substrate interaction involves the substrate binding to the enzyme's active site, forming an enzyme-substrate complex that facilitates the catalytic reaction. The holoenzyme, consisting of the apoenzyme and cofactors, provides the complete functional structure necessary for substrate binding and catalysis. This interaction is highly specific, driven by molecular complementarity and conformational changes that stabilize the transition state and lower the activation energy.
The Importance of Cofactors in Holoenzyme Formation
Cofactors play a crucial role in holoenzyme formation by enabling the enzymatic activity that substrates alone cannot achieve. Unlike substrates, which are specific reactants converted by enzymes, cofactors such as metal ions or organic molecules are essential for stabilizing the active site and facilitating catalysis within the holoenzyme complex. The absence of cofactors results in an inactive apoenzyme, highlighting their importance in biochemical reactions and efficient substrate processing.
Substrate Binding and Catalytic Activity
Substrate binding occurs when the substrate attaches specifically to the active site of an enzyme, initiating the catalytic process, whereas a holoenzyme represents the complete, active form of an enzyme including its protein component and necessary cofactors. The holoenzyme's structure facilitates optimal substrate binding and catalytic activity by stabilizing the substrate-enzyme complex and enabling precise catalytic mechanisms. This integration enhances the enzyme's efficiency, specificity, and reaction rate compared to the apoenzyme or substrate alone.
Real-World Examples: Substrates and Holoenzymes in Biology
Substrates such as glucose and ATP are essential molecules that enzymes act upon during metabolic processes like glycolysis and cellular respiration. Holoenzymes, including DNA polymerase and RNA polymerase, consist of apoenzymes combined with necessary cofactors or coenzymes, enabling catalytic activity essential for DNA replication and transcription. The interaction between substrates and holoenzymes drives critical biochemical reactions fundamental to cellular function and energy production.
Key Differences: Substrate vs Holoenzyme Comparison Table
Substrates are specific molecules upon which enzymes act to catalyze biochemical reactions, whereas holoenzymes are complete, catalytically active enzyme complexes comprising an apoenzyme and its necessary cofactors or coenzymes. Key differences include that substrates are reactants transformed during enzymatic reactions, while holoenzymes represent the functional form of the enzyme required for activity. The comparison table highlights substrates as ligands interacting with enzymes and holoenzymes as multicomponent assemblies essential for enzymatic catalysis and regulation.
Biological Significance and Applications
The substrate serves as the specific molecule upon which an enzyme acts, essential for facilitating biochemical reactions and metabolic processes critical to cellular function. The holoenzyme, comprising both the apoenzyme and its necessary cofactor, represents the active form of the enzyme, enabling precise catalytic activity and regulation of metabolic pathways. Understanding the interplay between substrates and holoenzymes informs drug design, enzymatic therapy, and biotechnological applications such as substrate-specific biosensors and enzyme-based industrial catalysts.
Frequently Asked Questions on Substrate and Holoenzyme
Substrate refers to the specific molecule upon which an enzyme acts to catalyze a chemical reaction, while a holoenzyme is the complete, active form of an enzyme consisting of the apoenzyme and its necessary cofactors or coenzymes. Frequently asked questions differentiate substrates as reactants in enzymatic reactions, whereas holoenzymes are discussed in terms of their structural components and functional roles. Understanding substrate specificity and holoenzyme assembly is essential for grasping enzyme mechanisms and biochemical pathways.
Substrate Infographic
 libterm.com
libterm.com