A solid foundation, or base, is essential for ensuring stability and durability in any structure or concept. Whether referring to physical constructions, business strategies, or personal skills, establishing a reliable base supports long-term success and growth. Explore the article to discover how building the right base can transform your outcomes and provide lasting benefits.
Table of Comparison
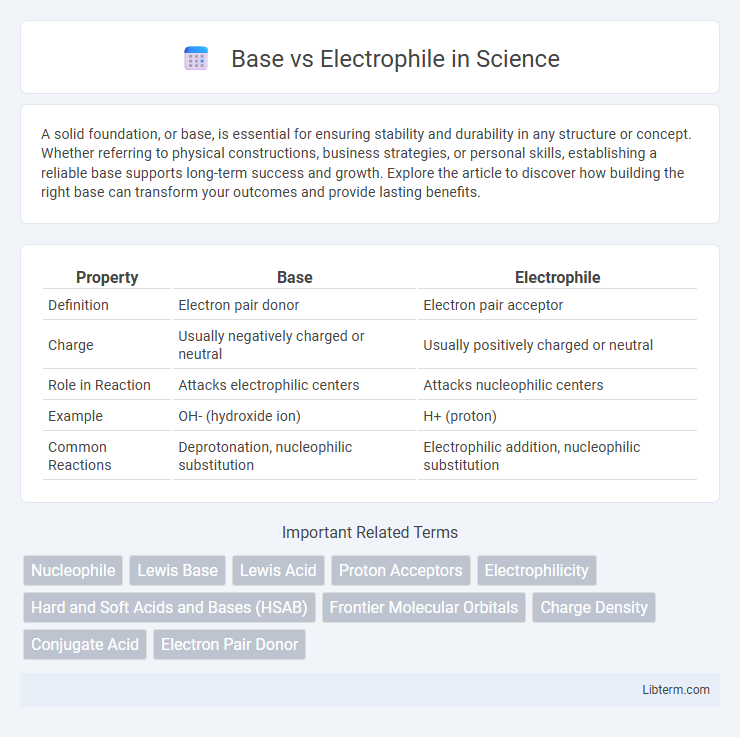
| Property | Base | Electrophile |
|---|---|---|
| Definition | Electron pair donor | Electron pair acceptor |
| Charge | Usually negatively charged or neutral | Usually positively charged or neutral |
| Role in Reaction | Attacks electrophilic centers | Attacks nucleophilic centers |
| Example | OH- (hydroxide ion) | H+ (proton) |
| Common Reactions | Deprotonation, nucleophilic substitution | Electrophilic addition, nucleophilic substitution |
Introduction to Bases and Electrophiles
Bases are chemical species that donate electron pairs and often possess lone pairs or negative charge, making them nucleophilic and prone to attack electrophilic centers. Electrophiles are electron-deficient species that accept electron pairs, typically characterized by positive charges or partial positive regions, enabling them to react with nucleophiles like bases. Understanding the interaction between bases and electrophiles is fundamental to predicting reaction mechanisms and the formation of chemical bonds in organic and inorganic chemistry.
Fundamental Definitions: Base vs Electrophile
A base is a substance that donates an electron pair to form a covalent bond, often characterized by its nucleophilicity and ability to accept protons or Lewis acids. An electrophile is an atom or molecule that accepts an electron pair from a nucleophile during a chemical reaction, typically featuring an electron-deficient center. Understanding the fundamental difference between bases and electrophiles is crucial for predicting reaction mechanisms and the direction of electron flow in organic chemistry.
Electronic Structure and Reactivity
Bases possess lone electron pairs or p electrons allowing them to donate electrons, stabilizing positively charged centers during reactions. Electrophiles feature electron-deficient atoms or positively polarized centers, making them susceptible to nucleophilic attack due to incomplete octets or positive formal charges. The electronic structure dictates reactivity: bases act as nucleophiles by providing electron density, while electrophiles seek electron density to complete their valence shells.
Lewis and Brønsted Concepts
Bases are defined by their ability to donate electron pairs in Lewis theory, acting as electron pair donors, while electrophiles are electron pair acceptors that seek electron density to form new bonds. In Bronsted-Lowry terms, bases are proton acceptors and electrophiles often correlate with acidic species that accept electrons as well as protons. Understanding the distinction between Lewis bases and Bronsted bases helps explain diverse reaction mechanisms involving nucleophiles and electrophiles in organic and inorganic chemistry.
Mechanisms of Base-Driven Reactions
In base-driven reactions, the base functions by donating electron pairs to abstract protons or stabilize negative charges, initiating nucleophilic attacks on electrophilic centers. Mechanisms such as E2 eliminations involve bases removing b-hydrogens, leading to the formation of alkenes through concerted proton abstraction and leaving group departure. Understanding the interplay between base strength, substrate structure, and electrophilic site electrophilicity is critical for predicting reaction pathways and product distributions in organic synthesis.
Typical Electrophilic Reaction Pathways
Typical electrophilic reaction pathways involve the interaction of an electrophile, a species seeking electrons, with a nucleophile or a base that donates electron density. Electrophiles commonly undergo addition, substitution, and elimination reactions depending on the substrate and reaction conditions, often facilitated by Lewis acids or protic acids enhancing electrophilic character. Key examples include the electrophilic aromatic substitution where electrophiles attack aromatic rings, and nucleophilic addition to carbonyl groups where electrophiles like carbocations drive the reaction forward.
Examples of Common Bases and Electrophiles
Common bases include hydroxide ion (OH-), ammonia (NH3), and alkoxides such as methoxide (CH3O-), which readily donate electron pairs to accept protons or react with electrophiles. Typical electrophiles feature positively polarized atoms or groups, like carbocations (R3C+), carbonyl carbon in aldehydes and ketones, and alkyl halides (R-X), which attract nucleophilic attack from bases. Understanding these examples aids in predicting reaction mechanisms in organic synthesis and catalysis.
Base vs Electrophile: Key Differences
Bases are electron pair donors that seek positively charged or electron-deficient sites, whereas electrophiles are electron pair acceptors that target electron-rich regions in molecules. Key differences include bases typically having lone pairs or negative charges, while electrophiles possess partial positive charges or vacant orbitals. Understanding their distinct roles is essential in predicting reaction mechanisms and outcomes in organic and inorganic chemistry.
Roles in Organic Synthesis
Bases in organic synthesis function primarily as proton acceptors, facilitating deprotonation to generate reactive intermediates like carbanions crucial for nucleophilic substitution and elimination reactions. Electrophiles act as electron-deficient species that accept electron pairs from nucleophiles, enabling bond formation through mechanisms such as nucleophilic addition and substitution. The interplay of bases and electrophiles drives key synthetic transformations, dictating reaction pathways and product outcomes in organic chemistry.
Applications in Industrial and Laboratory Chemistry
Bases are essential in industrial processes such as ammonia synthesis and biodiesel production, where they facilitate deprotonation and nucleophilic substitution reactions. Electrophiles are crucial in laboratory synthesis for forming carbon-carbon bonds through electrophilic addition and substitution, enabling complex molecule construction. Both bases and electrophiles serve complementary roles in catalysis and organic transformations, driving efficiency and selectivity in chemical manufacturing.
Base Infographic
 libterm.com
libterm.com