Hexose sugars are six-carbon monosaccharides essential in various biological processes, including energy production and cellular structure. Common examples include glucose, fructose, and galactose, each playing distinct roles in metabolism and nutrition. Discover how hexoses impact your health and the vital functions they serve by reading the full article.
Table of Comparison
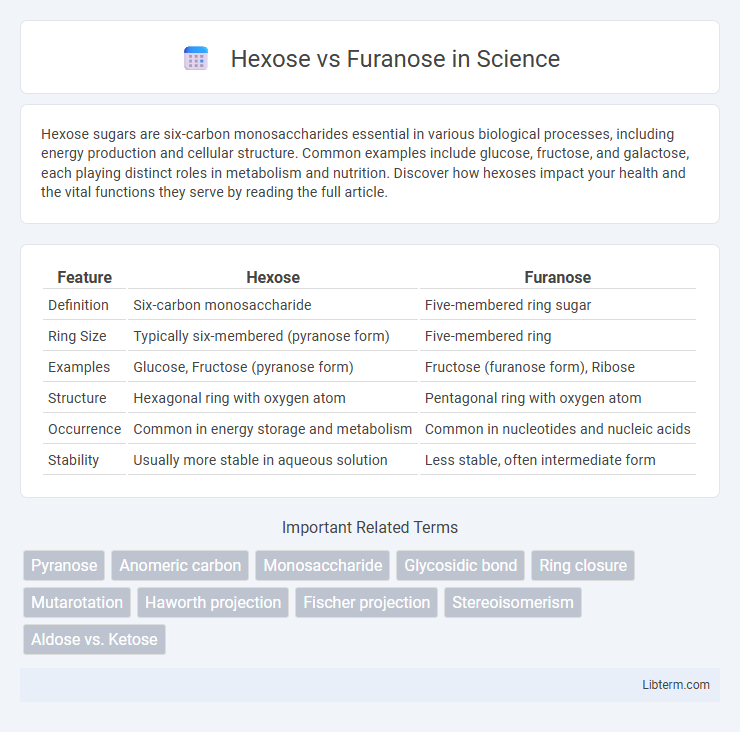
| Feature | Hexose | Furanose |
|---|---|---|
| Definition | Six-carbon monosaccharide | Five-membered ring sugar |
| Ring Size | Typically six-membered (pyranose form) | Five-membered ring |
| Examples | Glucose, Fructose (pyranose form) | Fructose (furanose form), Ribose |
| Structure | Hexagonal ring with oxygen atom | Pentagonal ring with oxygen atom |
| Occurrence | Common in energy storage and metabolism | Common in nucleotides and nucleic acids |
| Stability | Usually more stable in aqueous solution | Less stable, often intermediate form |
Introduction to Hexose and Furanose
Hexoses are six-carbon monosaccharides that serve as fundamental energy sources and structural components in biological systems. Furanoses are cyclic forms of sugars, including hexoses, characterized by a five-membered ring containing four carbons and one oxygen atom. The interconversion between linear hexoses and their furanose ring structures plays a critical role in carbohydrate chemistry and metabolism.
Structural Differences Between Hexose and Furanose
Hexoses are six-carbon sugars typically found in an open-chain or cyclic pyranose form, characterized by a six-membered ring consisting of five carbon atoms and one oxygen atom. Furanoses are five-membered ring structures containing four carbon atoms and one oxygen atom, commonly formed by the intramolecular reaction between a carbonyl group and a hydroxyl group within the sugar molecule. The key structural difference lies in the ring size and composition: hexoses predominantly form six-membered (pyranose) rings, whereas furanoses form five-membered rings, influencing their chemical reactivity and biological function.
Chemical Properties of Hexose vs Furanose
Hexose is a six-carbon monosaccharide with a linear or cyclic structure, typically forming a six-membered ring known as pyranose, whereas furanose refers to a five-membered ring structure formed when a hexose cyclizes through an internal reaction between the carbonyl and hydroxyl group. The chemical properties of hexoses include higher stability in their pyranose form, greater propensity for forming glycosidic bonds, and aldehyde or ketone functional groups that participate in redox reactions. Furanoses exhibit increased ring strain and reactivity due to their five-membered ring, often leading to distinct reactivity patterns in enzymatic recognition and glycosidic linkage formation compared to hexoses in pyranose form.
Nomenclature and Classification
Hexoses are six-carbon monosaccharides classified based on the position of their carbonyl group into aldoses or ketoses, such as glucose (an aldohexose) and fructose (a ketohexose). Furanoses refer to five-membered ring forms of monosaccharides, typically formed when a hexose's carbonyl carbon reacts with a hydroxyl group, resulting in a hemiacetal or hemiketal ring structure. The nomenclature of furanoses incorporates the ring form designation (furanose) along with the original hexose name, for example, a-D-glucopyranose versus b-D-fructofuranose, indicating ring size and stereochemistry.
Biological Role of Hexose
Hexoses, including glucose and fructose, serve as primary energy sources in biological systems through glycolysis and cellular respiration. Their linear and cyclic forms enable efficient metabolism and energy storage, with hexoses predominantly existing as pyranoses in physiological conditions. Unlike furanoses, which are five-membered rings mainly found in nucleotides, hexoses play a crucial role in energy supply, structural components of cells, and as intermediates in key biosynthetic pathways.
Biological Importance of Furanose
Furanose is a five-membered ring form of sugar crucial in biology, especially as a structural component of nucleotides and nucleic acids like RNA and DNA, where ribose and deoxyribose sugars predominantly adopt the furanose form. Unlike hexoses, which primarily exist as six-membered pyranose rings in solution and serve mainly as energy sources, furanoses contribute to molecular recognition, enzymatic activity, and genetic information storage. The biological importance of furanose lies in its role in forming the sugar-phosphate backbone of genetic material, enabling stable yet flexible structures essential for life processes.
Occurrence in Nature: Hexose and Furanose Examples
Hexoses commonly occur in nature as six-carbon sugars like glucose and fructose, which are vital for energy metabolism in plants and animals. Furanose forms are cyclic structures found in sugars such as ribose and fructose, where the sugar ring includes five atoms, including four carbons and one oxygen. Ribose, a key component of RNA, exists naturally in its furanose form, while glucose predominantly exists as a hexose in its pyranose form but can also adopt a minor furanose form under specific conditions.
Functional Implications in Biochemistry
Hexose sugars exist primarily in two structural forms: the open-chain and cyclic forms, with the cyclic forms being either pyranose (six-membered ring) or furanose (five-membered ring) rings. Furanose forms, such as beta-D-fructofuranose, exhibit distinct biochemical properties influencing enzyme specificity, glycosidic bond formation, and molecular recognition compared to the more stable pyranose forms seen in glucose. These structural differences impact metabolic pathways, including glycolysis and glycosylation, thus affecting energy production and cellular communication.
Analytical Methods for Differentiating Hexose and Furanose
Analytical methods for differentiating hexose and furanose structures primarily utilize nuclear magnetic resonance (NMR) spectroscopy, which identifies distinct chemical shifts corresponding to the five-membered furanose ring versus the six-membered hexose ring. Mass spectrometry (MS), coupled with chromatographic techniques like gas chromatography (GC) or liquid chromatography (LC), enables separation and identification based on ring size and fragmentation patterns unique to furanose or hexose conformations. Infrared (IR) spectroscopy also provides complementary data by detecting characteristic vibrational modes influenced by the ring structure, helping differentiate between hexose and furanose isomers in complex carbohydrate mixtures.
Summary: Key Differences Between Hexose and Furanose
Hexose refers to a six-carbon sugar molecule, while furanose describes a five-membered ring structure commonly formed by certain sugars, including some hexoses. Hexoses can exist in both linear and cyclic forms, with furanose being one type of cyclic conformation characterized by a ring containing four carbons and one oxygen atom. The primary difference lies in hexose defining the sugar's carbon count, whereas furanose specifies the ring size and structure within cyclic forms.
Hexose Infographic
 libterm.com
libterm.com