Superhydrophobic surfaces repel water effectively, creating a protective barrier that prevents liquid from adhering. This property enhances durability and cleanliness in various applications, from textiles to electronics. Discover how superhydrophobic technology can improve Your everyday products by reading the full article.
Table of Comparison
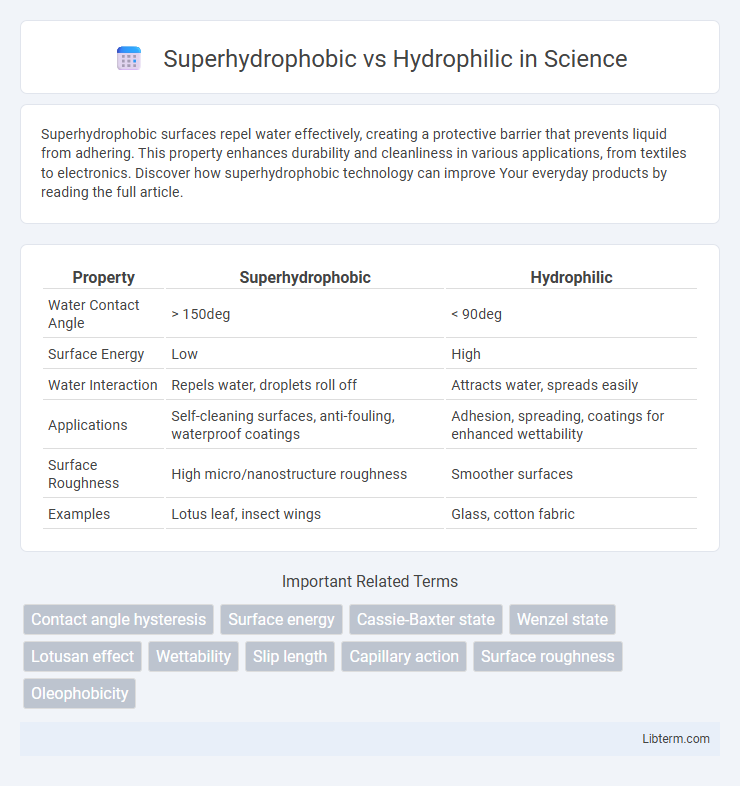
| Property | Superhydrophobic | Hydrophilic |
|---|---|---|
| Water Contact Angle | > 150deg | < 90deg |
| Surface Energy | Low | High |
| Water Interaction | Repels water, droplets roll off | Attracts water, spreads easily |
| Applications | Self-cleaning surfaces, anti-fouling, waterproof coatings | Adhesion, spreading, coatings for enhanced wettability |
| Surface Roughness | High micro/nanostructure roughness | Smoother surfaces |
| Examples | Lotus leaf, insect wings | Glass, cotton fabric |
Introduction to Surface Wettability
Surface wettability describes how liquids interact with solid surfaces, characterized primarily by superhydrophobic and hydrophilic properties. Superhydrophobic surfaces exhibit water contact angles greater than 150deg, causing water droplets to bead up and roll off easily, enhancing self-cleaning and corrosion resistance. In contrast, hydrophilic surfaces have contact angles less than 90deg, promoting water spread and adhesion, which is crucial for applications like coating adhesion and enhanced heat transfer.
What is Superhydrophobicity?
Superhydrophobicity refers to the extreme water-repellent property of a surface, characterized by water contact angles greater than 150 degrees, causing water droplets to bead up and roll off easily. This phenomenon results from a combination of low surface energy materials and micro- or nanoscale surface roughness, minimizing liquid-solid contact. In contrast, hydrophilic surfaces exhibit high surface energy and low contact angles, promoting water spreading and adhesion.
What is Hydrophilicity?
Hydrophilicity refers to the chemical property of a substance that enables it to interact favorably with water through hydrogen bonding or polarity, resulting in strong water adhesion and spreading. Materials exhibiting hydrophilicity have surfaces with high surface energy, which promotes water absorption and solubility, commonly seen in cellulose, silica, and certain polymers. This contrasts with superhydrophobic surfaces that repel water and minimize contact, making hydrophilicity crucial for applications like biomedical devices, coatings, and filtration systems.
Key Differences Between Superhydrophobic and Hydrophilic Surfaces
Superhydrophobic surfaces exhibit water contact angles greater than 150deg, causing water droplets to bead up and roll off easily, whereas hydrophilic surfaces have contact angles below 90deg, allowing water to spread and adhere. Superhydrophobicity results from micro- and nanoscale surface roughness combined with low surface energy materials, while hydrophilic surfaces typically feature high surface energy and smooth textures. These differences impact applications such as self-cleaning coatings, water filtration, and anti-corrosion treatments, highlighting the importance of surface characteristics in controlling wettability.
Mechanisms Behind Superhydrophobic Surfaces
Superhydrophobic surfaces achieve extreme water repellency through a combination of micro/nanoscale roughness and low surface energy materials, which trap air pockets and minimize the solid-liquid contact area. This phenomenon is often explained by the Cassie-Baxter model, where water droplets rest on a composite surface of solid and air, leading to high contact angles typically above 150 degrees. In contrast, hydrophilic surfaces exhibit strong affinity to water molecules due to higher surface energy and smoother textures, promoting spreading and absorption.
Mechanisms Behind Hydrophilic Surfaces
Hydrophilic surfaces attract and bind water molecules through polar or charged functional groups, enabling strong intermolecular hydrogen bonding. This interaction lowers the contact angle below 90 degrees, facilitating water spread and surface wetting. Surface energy and chemical composition, such as hydroxyl or carboxyl groups, play critical roles in enhancing hydrophilicity and promoting aqueous adhesion.
Practical Applications of Superhydrophobic Materials
Superhydrophobic materials exhibit extreme water repellency with contact angles exceeding 150deg, enabling applications such as self-cleaning surfaces, anti-icing coatings, and corrosion resistance in industries like aerospace, electronics, and textiles. These materials minimize water adhesion, reducing maintenance costs and enhancing durability in outdoor equipment and infrastructure. In contrast, hydrophilic surfaces attract water, promoting spreading and absorption useful in applications like anti-fogging, oil-water separation, and biomedical devices.
Practical Uses of Hydrophilic Materials
Hydrophilic materials attract and absorb water, making them ideal for applications such as wound dressings, contact lenses, and moisture-wicking fabrics. These materials facilitate efficient water transport, enhancing comfort and hygiene in medical and textile industries. The contrast with superhydrophobic surfaces, which repel water, highlights hydrophilic materials' critical role in ensuring moisture management and biocompatibility.
Advantages and Limitations of Each Surface Type
Superhydrophobic surfaces offer advantages such as self-cleaning properties, reduced corrosion, and enhanced water repellency, making them ideal for applications requiring moisture resistance and durability. However, their limitations include potential wear and degradation over time, complex manufacturing processes, and reduced efficacy under oily or contaminated conditions. Hydrophilic surfaces provide benefits like improved wettability, enhanced adhesion for coatings or biological applications, and efficient water spreading, but they may suffer from water retention issues, lower resistance to fouling, and decreased durability in harsh environments.
Future Trends in Surface Engineering
Future trends in surface engineering emphasize enhancing superhydrophobic coatings to improve durability, self-cleaning, and anti-corrosion properties in various industries, including automotive and aerospace. Innovations in nanotechnology and biomimetic designs aim to create surfaces exhibiting tunable wettability, allowing dynamic switching between superhydrophobic and hydrophilic states for applications in medical devices and smart textiles. Research into eco-friendly, scalable fabrication methods using advanced materials such as graphene and silicon nanowires continues to drive the development of multifunctional surfaces with improved environmental resistance and performance.
Superhydrophobic Infographic
 libterm.com
libterm.com