Hypotonic solutions have a lower concentration of solutes compared to the inside of a cell, causing water to flow into the cell and potentially leading to swelling or bursting. This concept is crucial in medical treatments and biological studies where cell volume and fluid balance must be carefully managed. Discover more about how hypotonic environments affect cells and their practical applications in the full article.
Table of Comparison
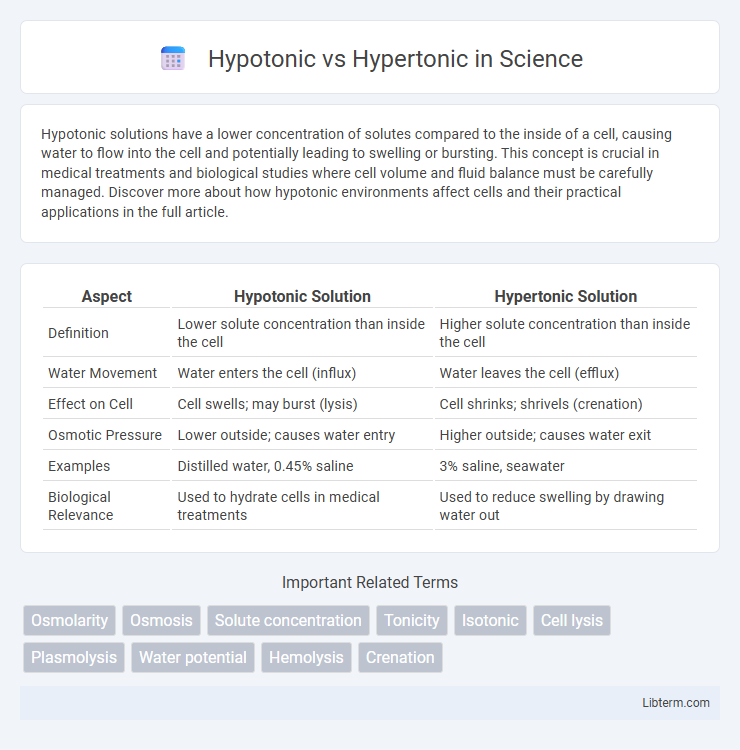
| Aspect | Hypotonic Solution | Hypertonic Solution |
|---|---|---|
| Definition | Lower solute concentration than inside the cell | Higher solute concentration than inside the cell |
| Water Movement | Water enters the cell (influx) | Water leaves the cell (efflux) |
| Effect on Cell | Cell swells; may burst (lysis) | Cell shrinks; shrivels (crenation) |
| Osmotic Pressure | Lower outside; causes water entry | Higher outside; causes water exit |
| Examples | Distilled water, 0.45% saline | 3% saline, seawater |
| Biological Relevance | Used to hydrate cells in medical treatments | Used to reduce swelling by drawing water out |
Understanding Hypotonic and Hypertonic Solutions
Hypotonic solutions have a lower concentration of solutes compared to the inside of a cell, causing water to flow into the cell and potentially leading to swelling or lysis. Hypertonic solutions contain a higher concentration of solutes than the cell's interior, resulting in water moving out of the cell, which can cause cell shrinkage or crenation. Understanding the osmotic balance between these solutions is critical in medical treatments like intravenous fluid administration to prevent cell damage.
Key Differences Between Hypotonic and Hypertonic
Hypotonic solutions have lower solute concentration compared to the cell's interior, causing water to flow into the cell and potentially leading to swelling or lysis. Hypertonic solutions contain higher solute concentration than the cell, resulting in water exiting the cell and causing shrinkage or crenation. The key difference lies in the direction of osmotic water movement driven by solute concentration gradients relative to the cell membrane.
Osmosis: The Science Behind Tonicity
Osmosis drives water movement across cell membranes, influenced by tonicities such as hypotonic and hypertonic solutions. In a hypotonic environment, water flows into the cell, causing swelling due to higher solute concentration inside the cell compared to the outside. Conversely, a hypertonic solution causes water to exit the cell, leading to cell shrinkage as the external solute concentration exceeds that within the cell.
Effects on Cells: Hypotonic vs Hypertonic
Hypotonic solutions cause cells to swell as water enters through osmosis, risking lysis or bursting due to increased internal pressure. Hypertonic solutions lead to cell shrinkage or crenation by drawing water out, resulting in dehydration and impaired cell function. Understanding these effects is crucial for medical treatments like intravenous fluid administration and cellular osmotic balance.
Clinical Applications in Medicine
Hypotonic solutions, such as 0.45% saline, are used in clinical settings to treat cellular dehydration by promoting water movement into cells, essential in conditions like hypernatremia and diabetic ketoacidosis. Hypertonic solutions, like 3% saline, are employed to reduce cerebral edema and manage increased intracranial pressure by drawing fluid out of swollen brain cells into the vascular space. Understanding the tonicity of intravenous fluids is critical in managing electrolyte imbalances and preventing cellular damage in patients with severe dehydration or edema.
Common Examples in Daily Life
Hypotonic solutions, such as distilled water and fruit juices, have lower solute concentrations compared to body fluids, causing cells to swell when exposed. Hypertonic solutions like seawater, saline nasal sprays, and certain sports drinks contain higher solute levels, leading to cell shrinkage as water exits the cells. These everyday substances illustrate the critical role of solute concentration in fluid balance and cellular function.
Risks and Benefits of Each Solution
Hypotonic solutions, containing lower solute concentration than blood, help rehydrate cells and treat conditions like hypernatremia but may cause cellular swelling and risk of hemolysis if used excessively. Hypertonic solutions have higher solute concentration than blood and effectively reduce cerebral edema and stabilize blood pressure by drawing fluid out of cells, yet they carry risks of causing cellular dehydration, electrolyte imbalances, and vein irritation. Careful monitoring of fluid status and electrolyte levels ensures the safe and effective use of both hypotonic and hypertonic IV therapies.
Identifying Hypotonic and Hypertonic Solutions
Hypotonic solutions have a lower solute concentration compared to the inside of the cell, causing water to move into the cell and potentially leading to swelling or lysis. Hypertonic solutions contain a higher solute concentration than the cell's interior, resulting in water moving out of the cell, which causes cell shrinkage or crenation. Identifying hypotonic and hypertonic solutions involves comparing the osmolarity of the solution to the cytoplasm, where hypotonic is less than and hypertonic is greater than the cell's internal solute concentration.
Lab Testing: Determining Tonicity
Lab testing for determining tonicity involves measuring the osmolarity of a solution compared to blood plasma, typically using an osmometer. Hypotonic solutions have lower osmolarity than plasma, causing cells to swell due to water influx, while hypertonic solutions exhibit higher osmolarity, leading to cell shrinkage as water exits. Accurate tonicity assessment is essential in clinical settings to guide intravenous fluid therapy and prevent cellular damage.
Frequently Asked Questions About Tonicity
Hypotonic solutions have lower solute concentration compared to the cell's interior, causing water to move into the cell and potentially leading to swelling or bursting. Hypertonic solutions contain higher solute concentrations than the cell, resulting in water moving out of the cell and causing it to shrink or crenate. Tonicity frequently addresses how cells respond to different solutions, osmotic pressure differences, and the impact on cell volume and function in medical and biological contexts.
Hypotonic Infographic
 libterm.com
libterm.com