Isomers are molecules with the same molecular formula but different structures, affecting their chemical properties and reactivity. Allotropes refer to different structural forms of the same element, such as carbon existing as diamond, graphite, and graphene, each with unique physical characteristics. Discover how understanding isomers and allotropes can enhance your grasp of chemistry by reading the rest of the article.
Table of Comparison
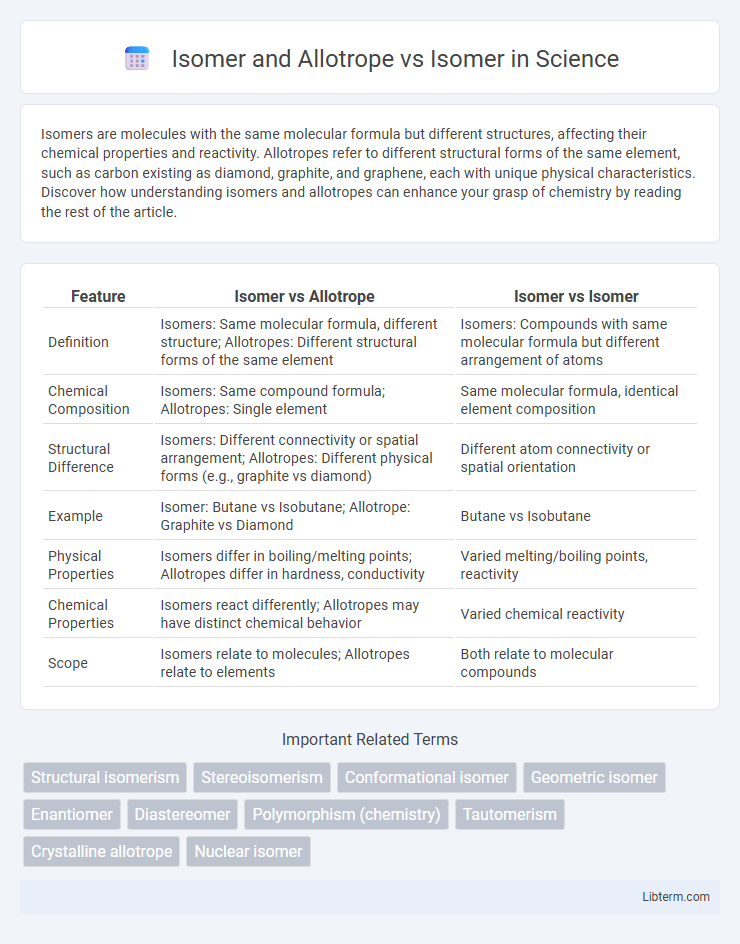
| Feature | Isomer vs Allotrope | Isomer vs Isomer |
|---|---|---|
| Definition | Isomers: Same molecular formula, different structure; Allotropes: Different structural forms of the same element | Isomers: Compounds with same molecular formula but different arrangement of atoms |
| Chemical Composition | Isomers: Same compound formula; Allotropes: Single element | Same molecular formula, identical element composition |
| Structural Difference | Isomers: Different connectivity or spatial arrangement; Allotropes: Different physical forms (e.g., graphite vs diamond) | Different atom connectivity or spatial orientation |
| Example | Isomer: Butane vs Isobutane; Allotrope: Graphite vs Diamond | Butane vs Isobutane |
| Physical Properties | Isomers differ in boiling/melting points; Allotropes differ in hardness, conductivity | Varied melting/boiling points, reactivity |
| Chemical Properties | Isomers react differently; Allotropes may have distinct chemical behavior | Varied chemical reactivity |
| Scope | Isomers relate to molecules; Allotropes relate to elements | Both relate to molecular compounds |
Introduction to Isomers and Allotropes
Isomers are molecules with the same molecular formula but different structural arrangements, impacting their chemical and physical properties significantly. Allotropes represent different structural forms of the same element in the same physical state, like carbon's diamond and graphite, which differ in atomic arrangement. Understanding the distinction between isomers and allotropes is crucial for grasping molecular diversity and elemental variation in chemistry.
Defining Isomers: Types and Examples
Isomers are molecules with the same molecular formula but different structures, including structural isomers, which differ in connectivity, and stereoisomers, which differ in spatial arrangement. Allotropes are different forms of the same element, such as graphite and diamond for carbon, distinct from isomers as they involve elemental forms rather than molecular structures. Examples of isomers include glucose and fructose (structural isomers) and cis- and trans-2-butene (stereoisomers), illustrating the diversity within isomer types.
Understanding Allotropes: Key Concepts
Allotropes are different structural forms of the same element in the same physical state, such as carbon existing as diamond, graphite, and graphene, each with distinct atomic arrangements and properties. Unlike isomers, which refer to compounds with the same molecular formula but different connectivity or spatial arrangement, allotropes highlight variations in elemental bonding configurations. Understanding allotropes is essential for materials science and chemistry as they influence physical properties, reactivity, and applications of elements.
Isomer vs Allotrope: Core Differences
Isomers are molecules with the same molecular formula but different structural arrangements, leading to distinct chemical properties, whereas allotropes are different structural forms of the same element in the same physical state, such as carbon existing as diamond, graphite, or graphene. Isomers primarily pertain to compounds, showcasing variations like structural, geometric, and optical isomerism, while allotropes demonstrate variations in elemental bonding and atomic arrangement within a pure element. The core difference lies in isomers involving different compounds with identical formulas, and allotropes involving different physical or chemical forms of a single element.
Structural Isomers vs Allotropes
Structural isomers are molecules with the same molecular formula but different connectivity of atoms, resulting in distinct chemical properties, while allotropes are different structural forms of the same element exhibiting varied physical and chemical characteristics. Unlike allotropes, which involve variations in atomic arrangement within a single element, structural isomers pertain to compounds composed of multiple elements with differing bonding patterns. Understanding the differences between structural isomers and allotropes is essential for fields like organic chemistry and materials science, where molecular structure influences functionality and reactivity.
Functional Isomers and Allotrope Comparison
Functional isomers differ in the type of functional groups within molecules sharing the same molecular formula, leading to distinct chemical properties and reactivity patterns. Allotropes represent different structural forms of a single element, such as carbon's diamond and graphite, characterized by varying atomic arrangements and physical properties. Comparing functional isomers to allotropes highlights that isomers emphasize variations in molecular connectivity and function, whereas allotropes reflect alterations in elemental bonding and structural organization.
Physical Properties: Isomers vs Allotropes
Isomers are compounds with the same molecular formula but different structural arrangements, resulting in distinct physical properties such as melting point, boiling point, and solubility. Allotropes are different structural forms of the same element, like graphite and diamond for carbon, exhibiting unique physical characteristics including hardness, electrical conductivity, and density. The key difference lies in isomers being molecules with varied connectivity of atoms, while allotropes represent different atomic arrangements within a single element affecting their physical states.
Chemical Properties: Contrasting Isomers and Allotropes
Isomers exhibit differences in chemical properties primarily due to variations in atomic connectivity or spatial arrangement, affecting reactivity and interaction with other molecules. Allotropes, on the other hand, consist of the same element in distinct structural forms, leading to vastly different chemical behaviors despite identical elemental composition. The contrasting chemical properties of isomers and allotropes highlight the influence of molecular structure versus elemental arrangement on substance reactivity.
Real-World Applications: Isomers and Allotropes
Isomers and allotropes play crucial roles in various industries due to their unique structural variations and properties. Isomers, such as cis-trans or enantiomers, impact pharmaceuticals by influencing drug efficacy and metabolism, while allotropes of carbon like graphite and diamond find applications in electronics, cutting tools, and lubricants because of their differing atomic arrangements. Understanding these differences enables advancements in material science, medicine, and chemical manufacturing.
Summary: Distinguishing Isomers from Allotropes
Isomers are molecules with the same molecular formula but different structural arrangements or spatial orientations, resulting in distinct chemical properties. Allotropes are different structural forms of the same element, such as carbon existing as diamond, graphite, or graphene, with varying physical and chemical characteristics. The key distinction lies in isomers involving compounds with identical atoms arranged differently, while allotropes refer specifically to elemental forms demonstrating diverse bonding and structure.
Isomer and Allotrope Infographic
 libterm.com
libterm.com