Isomers are compounds with the same molecular formula but different arrangements of atoms, resulting in distinct chemical properties. Conformers are a subset of isomers that differ only by rotation around single bonds, representing different spatial orientations of the same molecule. Explore the rest of the article to understand how these differences impact your study or application in chemistry.
Table of Comparison
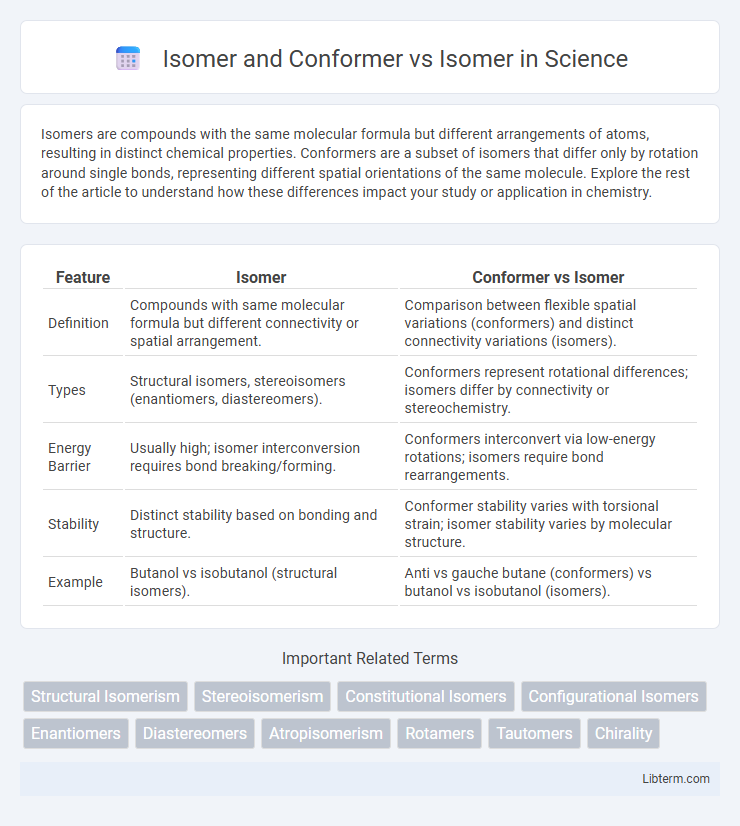
| Feature | Isomer | Conformer vs Isomer |
|---|---|---|
| Definition | Compounds with same molecular formula but different connectivity or spatial arrangement. | Comparison between flexible spatial variations (conformers) and distinct connectivity variations (isomers). |
| Types | Structural isomers, stereoisomers (enantiomers, diastereomers). | Conformers represent rotational differences; isomers differ by connectivity or stereochemistry. |
| Energy Barrier | Usually high; isomer interconversion requires bond breaking/forming. | Conformers interconvert via low-energy rotations; isomers require bond rearrangements. |
| Stability | Distinct stability based on bonding and structure. | Conformer stability varies with torsional strain; isomer stability varies by molecular structure. |
| Example | Butanol vs isobutanol (structural isomers). | Anti vs gauche butane (conformers) vs butanol vs isobutanol (isomers). |
Introduction to Isomers
Isomers are molecules with the same molecular formula but different arrangements of atoms, resulting in distinct chemical properties. Isomers are broadly classified into structural isomers, which differ in connectivity, and stereoisomers, which have the same connectivity but vary in spatial orientation. Conformers, a subtype of stereoisomers, differ only by rotation around single bonds, whereas isomers encompass all structural and stereochemical variations.
What Are Conformers?
Conformers are a type of isomer characterized by different spatial orientations due to rotation around single bonds without breaking any bonds, distinguishing them from other isomers that differ in connectivity. These rotational arrangements result in unique three-dimensional shapes impacting molecular properties such as reactivity and interaction with biological targets. Understanding conformers is crucial for drug design and materials science, as their energy differences and interconversion rates influence function and stability.
Types of Isomerism
Isomers are molecules with the same molecular formula but different arrangements of atoms, categorized into structural isomers and stereoisomers. Structural isomers differ in connectivity, including chain, position, and functional group isomerism, while conformers are a type of stereoisomer differing by rotation around single bonds without breaking bonds. Conformers represent different spatial orientations of the same molecule, making them a subset within stereoisomerism, contrasting with broader isomers that include both structural and stereochemical variations.
Structural Isomers vs Stereoisomers
Structural isomers differ in the connectivity of atoms within molecules, resulting in distinct arrangements of their chemical bonds, while stereoisomers share the same atomic connectivity but differ in the spatial orientation of their atoms. Among stereoisomers, conformers represent different rotational forms around single bonds that can interconvert rapidly, whereas configurational stereoisomers such as enantiomers and diastereomers cannot be interconverted without breaking bonds. Understanding the key differences between structural isomers and stereoisomers is essential for predicting molecular properties and reactivity in organic chemistry.
Conformational Isomerism Explained
Isomers are compounds with the same molecular formula but different structural arrangements, while conformers are a specific type of isomer differing only in the rotation around single bonds without breaking covalent bonds. Conformational isomerism, also known as rotamerism, involves these spatial variations, which significantly impact the molecule's physical and chemical properties despite having identical connectivity. This form of isomerism is crucial in understanding molecular flexibility, energy states, and reactions in organic chemistry and biochemistry.
Isomer vs Conformer: Key Differences
Isomers are compounds with the same molecular formula but different structural arrangements, while conformers are specific types of isomers differing only in the spatial orientation of atoms due to rotation around single bonds. Isomers include both structural isomers, which differ in connectivity, and stereoisomers, which share connectivity but differ in spatial arrangement; conformers fall under stereoisomers as rotational variants. Understanding the distinction between isomers and conformers is essential for predicting chemical reactivity and molecular behavior in fields like organic chemistry and drug design.
Significance of Isomers in Chemistry
Isomers, including both isomers and conformers, play a crucial role in chemistry as they exhibit identical molecular formulas but differ in structural arrangement or spatial orientation, profoundly influencing chemical properties and biological activity. Isomers can be classified into structural isomers, which differ in connectivity, and stereoisomers, which include conformers differing by rotation around single bonds. Understanding these variations is significant for drug design, material science, and reaction mechanisms, as small changes in isomer structure can lead to drastically different interactions and functionalities.
Real-world Examples of Isomers and Conformers
Isomers such as glucose and fructose illustrate structural isomerism, where compounds share molecular formulas but differ in atom connectivity, resulting in distinct biological functions. Conformers, exemplified by the chair and boat forms of cyclohexane, represent different spatial orientations of the same molecule that interconvert through rotation about single bonds, affecting stability and reactivity. These real-world examples highlight how isomers influence chemical properties and biological activity differently from conformers, which primarily impact molecular dynamics and conformational analysis.
Analytical Techniques for Isomer Identification
Isomers and conformers require advanced analytical techniques for precise identification, with isomers exhibiting different connectivity or arrangements that are distinguishable through methods like Nuclear Magnetic Resonance (NMR) spectroscopy and Mass Spectrometry (MS). Conformers, being rotational variants of the same molecule, are best analyzed using techniques such as Infrared (IR) spectroscopy and computational modeling to capture subtle energy differences and spatial configurations. Chromatographic methods like Gas Chromatography (GC) and Liquid Chromatography (LC) coupled with MS enhance the resolution and identification of isomeric species by separating molecules based on their physical and chemical properties.
Conclusion: Isomer and Conformer Distinctions
Isomers are compounds with the same molecular formula but different structural arrangements, leading to distinct physical and chemical properties. Conformers, a subset of isomers, differ only by rotations around single bonds and typically interconvert rapidly at room temperature. Understanding the distinctions between isomers and conformers is crucial for accurately predicting molecular behavior and reactivity in chemical and pharmaceutical applications.
Isomer and Conformer Infographic
 libterm.com
libterm.com