Lysogenic cycles allow viruses to integrate their genetic material into the host cell's DNA, remaining dormant for extended periods without destroying the host. This process influences bacterial evolution and gene transfer, often impacting your understanding of microbial behavior and viral infections. Explore the full article to uncover how lysogenic dynamics affect health and disease.
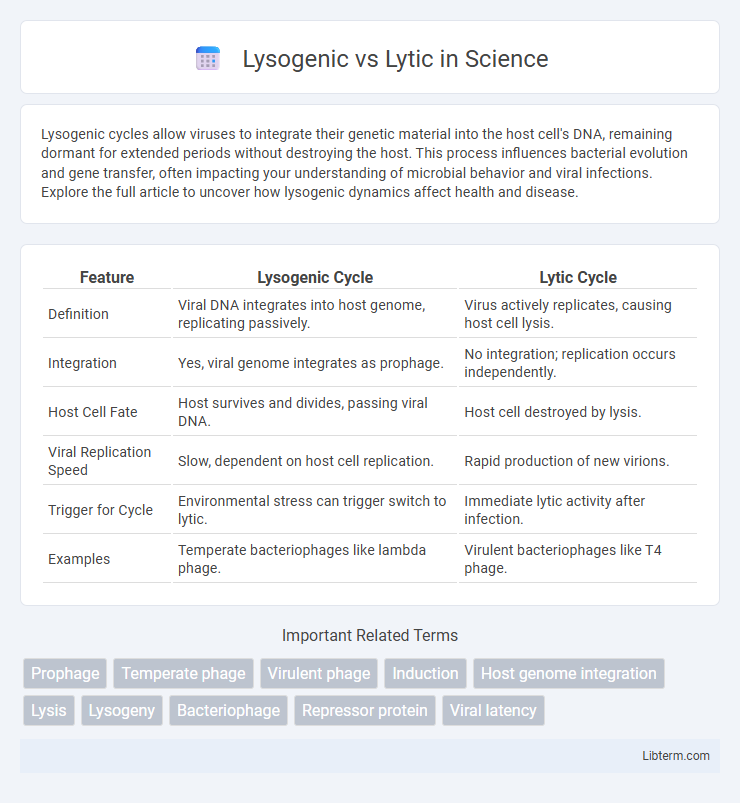
Table of Comparison
| Feature | Lysogenic Cycle | Lytic Cycle |
|---|---|---|
| Definition | Viral DNA integrates into host genome, replicating passively. | Virus actively replicates, causing host cell lysis. |
| Integration | Yes, viral genome integrates as prophage. | No integration; replication occurs independently. |
| Host Cell Fate | Host survives and divides, passing viral DNA. | Host cell destroyed by lysis. |
| Viral Replication Speed | Slow, dependent on host cell replication. | Rapid production of new virions. |
| Trigger for Cycle | Environmental stress can trigger switch to lytic. | Immediate lytic activity after infection. |
| Examples | Temperate bacteriophages like lambda phage. | Virulent bacteriophages like T4 phage. |
Introduction to Viral Replication Cycles
The lysogenic and lytic cycles represent two distinct viral replication strategies used by bacteriophages to propagate within host cells. In the lysogenic cycle, the viral DNA integrates into the host genome, remaining dormant until environmental triggers induce activation, whereas the lytic cycle involves immediate replication and assembly of new virions followed by host cell lysis and viral release. Understanding these cycles is crucial for studying viral pathogenesis, latency, and therapeutic interventions targeting viral infections.
Overview of Lysogenic Cycle
The lysogenic cycle involves the integration of a bacteriophage's genetic material into the host bacterium's genome, forming a prophage. This dormant state allows the viral DNA to replicate passively as the host cell divides, without causing immediate harm. Environmental triggers can induce the prophage to enter the lytic cycle, leading to the production of new phages and eventual host cell lysis.
Overview of Lytic Cycle
The lytic cycle involves the viral replication process where a bacteriophage infects a host cell, takes over its machinery to synthesize viral components, and assembles new viral particles. This cycle culminates in host cell lysis, releasing progeny viruses to infect neighboring cells. Key stages include attachment, genome injection, replication, assembly, and cell lysis.
Key Differences Between Lysogenic and Lytic Cycles
The lysogenic cycle integrates the phage DNA into the host bacterial genome, allowing the viral genome to replicate passively with the host cell without causing immediate harm. In contrast, the lytic cycle involves the active replication of the virus, culminating in host cell lysis and the release of new viral particles. Key differences include the lysogenic cycle's latency phase versus the lytic cycle's immediate destruction of the host cell.
Molecular Mechanisms of Lysogenic Cycle
The lysogenic cycle involves the integration of the bacteriophage genome into the host bacterium's DNA through site-specific recombination mediated by integrase enzymes, allowing the phage DNA to replicate passively with the host genome. Key regulatory proteins, such as the lambda repressor (cI protein), maintain the phage in a dormant prophage state by repressing lytic genes and preventing the expression of lytic enzymes. Environmental triggers like DNA damage activate the SOS response, leading to cleavage of the repressor and induction of the lytic cycle, where viral replication results in host cell lysis.
Molecular Mechanisms of Lytic Cycle
The molecular mechanisms of the lytic cycle involve the phage attaching to the bacterial surface and injecting its DNA into the host cell, followed by the immediate takeover of the host's replication machinery to transcribe and translate viral genes. Viral enzymes degrade the bacterial chromosome, allowing phage genomic replication and protein synthesis, which culminates in the assembly of new virions. Lysozyme-like enzymes then lyse the bacterial cell wall, releasing progeny phages to infect neighboring cells.
Examples of Viruses Using Lysogenic and Lytic Cycles
Bacteriophage lambda is a prime example of a virus that employs the lysogenic cycle, integrating its DNA into the host genome and replicating silently until triggered to enter the lytic phase. In contrast, bacteriophage T4 exemplifies the lytic cycle by rapidly hijacking the host's cellular machinery to produce new viral particles, culminating in cell lysis and release. Herpes simplex virus (HSV) also exhibits lysogeny-like latency, maintaining viral DNA in host neurons with periodic reactivation, while influenza virus follows a strictly lytic replication process, causing host cell destruction upon viral release.
Environmental Triggers for Lytic and Lysogenic Switch
Environmental triggers such as UV radiation, nutrient availability, and host cell stress influence the switch between lysogenic and lytic cycles in bacteriophages. DNA damage or metabolic stress often induce the prophage to enter the lytic cycle, promoting viral replication and host cell lysis. Stable conditions and host cell health favor maintenance of the lysogenic state, allowing viral DNA integration and dormancy.
Biological Implications and Consequences
Lysogenic and lytic cycles represent two distinct viral replication strategies with significant biological implications. Lysogenic viruses integrate their genome into the host DNA, allowing latent infection and genetic material transfer without immediate host cell destruction, which can lead to horizontal gene transfer and long-term host-virus coexistence. Lytic viruses rapidly replicate and lyse the host cell, causing acute infection and often triggering immune responses, influencing viral spread and host population dynamics.
Applications in Biotechnology and Medicine
Lysogenic and lytic cycles of bacteriophages offer distinct applications in biotechnology and medicine, where lysogenic phages are used in gene delivery systems for stable genetic modification and in developing phage therapy targeting antibiotic-resistant bacteria. Lytic phages are employed for their ability to rapidly lyse bacterial cells, making them effective antibacterial agents in phage therapy and biofilm disruption. Advances in synthetic biology harness these cycles to engineer phages with tailored host specificity and controlled lysis timing for precision antimicrobial treatments and diagnostic tools.
Lysogenic Infographic
 libterm.com
libterm.com