A leaving group is an atom or group of atoms that departs from a molecule during a chemical reaction, typically in substitution or elimination processes. Its ability to stabilize the negative charge after departure greatly influences the reaction rate and mechanism. Explore the article to understand how the nature of your leaving group affects reaction outcomes.
Table of Comparison
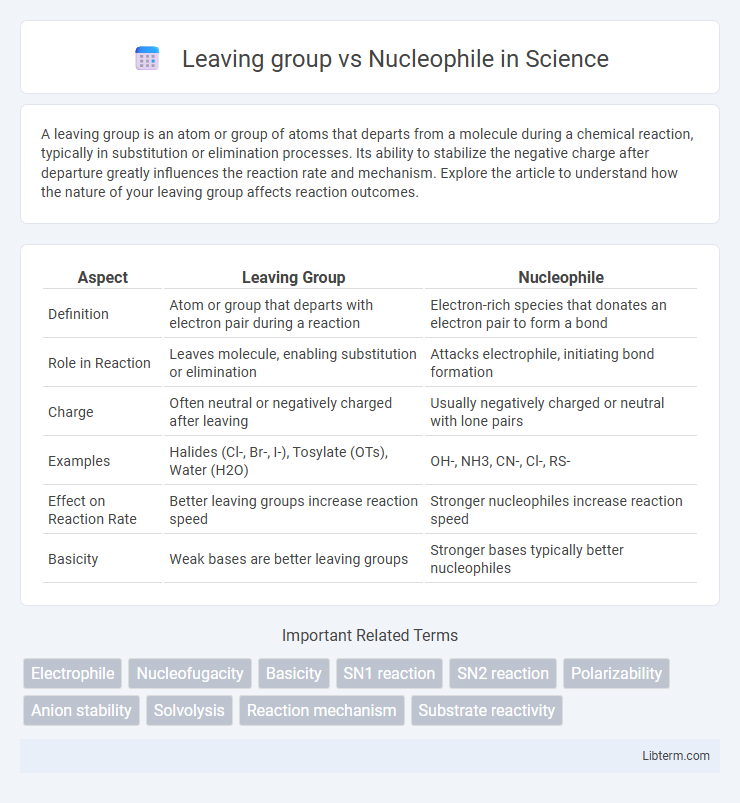
| Aspect | Leaving Group | Nucleophile |
|---|---|---|
| Definition | Atom or group that departs with electron pair during a reaction | Electron-rich species that donates an electron pair to form a bond |
| Role in Reaction | Leaves molecule, enabling substitution or elimination | Attacks electrophile, initiating bond formation |
| Charge | Often neutral or negatively charged after leaving | Usually negatively charged or neutral with lone pairs |
| Examples | Halides (Cl-, Br-, I-), Tosylate (OTs), Water (H2O) | OH-, NH3, CN-, Cl-, RS- |
| Effect on Reaction Rate | Better leaving groups increase reaction speed | Stronger nucleophiles increase reaction speed |
| Basicity | Weak bases are better leaving groups | Stronger bases typically better nucleophiles |
Introduction to Leaving Groups and Nucleophiles
Leaving groups are atoms or groups of atoms that detach from a molecule during a chemical reaction, facilitating the substitution or elimination process. Their ability to stabilize the negative charge after departure is crucial, with common leaving groups including halides like chloride and bromide. Nucleophiles, in contrast, are electron-rich species that donate a pair of electrons to form a new bond, varying widely in strength based on their charge, electronegativity, and solvent environment.
Fundamental Definitions
A leaving group is an atom or group of atoms that can depart with a pair of electrons during a chemical reaction, typically in substitution or elimination processes, facilitating bond cleavage. A nucleophile is an electron-rich species that donates an electron pair to form a new covalent bond with an electrophilic center. The fundamental distinction lies in the leaving group's ability to stabilize the negative charge after departure, while the nucleophile initiates bond formation by attacking an electron-deficient atom.
Key Characteristics of Leaving Groups
Leaving groups are atoms or groups that detach from the substrate during a substitution or elimination reaction, facilitating bond cleavage. Key characteristics of effective leaving groups include their ability to stabilize the negative charge after departure, often evidenced by weak basicity and resonance stabilization. Halides like iodide and bromide are classic examples, exhibiting good leaving group ability due to their size and polarizability.
Essential Traits of Nucleophiles
Nucleophiles are electron-rich species characterized by their ability to donate an electron pair to electrophiles during chemical reactions. Essential traits include high electron density, negative or partial negative charge, and availability of lone pairs or pi bonds for bond formation. Unlike leaving groups that detach from the substrate, nucleophiles actively seek positively charged or electron-deficient centers to initiate bond formation.
Role in Chemical Reactions
Leaving groups facilitate chemical reactions by departing from the substrate, stabilizing the negative charge after bond cleavage, which accelerates substitution or elimination processes. Nucleophiles act as electron pair donors, attacking electrophilic centers to form new chemical bonds during these reactions. The efficiency of leaving groups and the strength of nucleophiles directly influence reaction rates and mechanisms in organic synthesis.
Comparative Strength and Reactivity
Leaving groups are characterized by their stability once they depart with an electron pair, with weak bases like halides (I- > Br- > Cl-) typically serving as stronger leaving groups due to their low basicity and high polarizability. Nucleophiles exhibit varying strength depending on their charge, electronegativity, and solvent conditions, with negatively charged species generally acting as stronger nucleophiles compared to neutral analogs. The reactivity balance in substitution reactions hinges on a strong nucleophile effectively attacking the electrophilic center while a strong leaving group readily dissociates, facilitating reaction progress.
Influence on Reaction Mechanism
The leaving group's ability to stabilize the departing electron pair directly affects the reaction mechanism by determining whether a reaction proceeds via an SN1 or SN2 pathway. A strong leaving group, such as a halide ion (I-, Br-, Cl-), facilitates unimolecular nucleophilic substitution (SN1) by stabilizing the carbocation intermediate, while a poor leaving group favors a bimolecular concerted mechanism (SN2). The nucleophile's strength and steric bulk influence the rate and pathway by either promoting backside attack in SN2 or influencing carbocation stability in SN1 mechanisms.
Effects on Reaction Rate
The leaving group's stability significantly affects the reaction rate; better leaving groups, such as halides like iodide or tosylate ions, facilitate faster reactions by stabilizing the negative charge after departure. The nucleophile's strength also influences the rate, with stronger nucleophiles like hydroxide or cyanide ions increasing the reaction speed by more effectively attacking the electrophilic center. In SN1 reactions, the leaving group's ability is often the rate-determining factor, while in SN2 reactions, both leaving group quality and nucleophile strength critically impact the overall reaction rate.
Examples in Organic Synthesis
In organic synthesis, leaving groups such as tosylate (TsO-), bromide (Br-), and chloride (Cl-) facilitate substitution reactions by departing with electron pairs, allowing nucleophiles like hydroxide (OH-), cyanide (CN-), or amines (RNH2) to attack electrophilic centers. Strong leaving groups are typically weak bases, stabilizing the negative charge once detached, which is crucial for reaction rate and mechanism, exemplified by the use of iodide (I-) in SN2 reactions. Nucleophiles vary in strength and steric hindrance, influencing selectivity and product outcome, such as the use of azide ion (N3-) for nucleophilic substitution yielding azido derivatives in synthesis pathways.
Practical Applications and Considerations
Understanding the relationship between leaving groups and nucleophiles is crucial in organic synthesis, as the efficiency of substitution and elimination reactions depends on the leaving group's ability to depart and the nucleophile's strength. Good leaving groups, such as halides like bromide or tosylates, stabilize the negative charge after departure, facilitating smoother reaction pathways, while strong nucleophiles like hydroxide or alkoxide ions accelerate bond formation with electrophilic carbons. Practical applications include designing pharmaceuticals and agrochemicals where fine-tuning nucleophile and leaving group reactivity optimizes reaction yields and selectivity under specific conditions.
Leaving group Infographic
 libterm.com
libterm.com