Reduction in various contexts involves minimizing waste, costs, or negative impacts to enhance efficiency and sustainability. Implementing effective reduction strategies can lead to significant improvements in resource management and overall performance. Explore this article to discover practical approaches and benefits of reduction in your daily life and work.
Table of Comparison
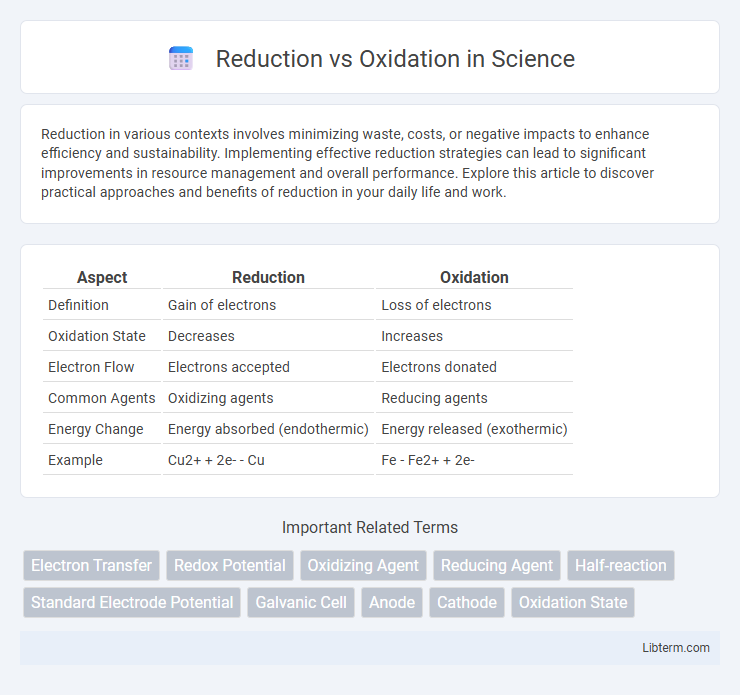
| Aspect | Reduction | Oxidation |
|---|---|---|
| Definition | Gain of electrons | Loss of electrons |
| Oxidation State | Decreases | Increases |
| Electron Flow | Electrons accepted | Electrons donated |
| Common Agents | Oxidizing agents | Reducing agents |
| Energy Change | Energy absorbed (endothermic) | Energy released (exothermic) |
| Example | Cu2+ + 2e- - Cu | Fe - Fe2+ + 2e- |
Understanding Redox Reactions
Redox reactions involve the transfer of electrons between substances, where reduction is the gain of electrons and oxidation is the loss of electrons. These reactions are fundamental in processes such as cellular respiration, photosynthesis, and corrosion. Understanding the electron flow in redox reactions helps explain energy transformations and chemical changes in various biological and industrial systems.
Definition of Reduction
Reduction is a chemical process characterized by the gain of electrons by an atom, ion, or molecule, leading to a decrease in oxidation state. This reaction often occurs simultaneously with oxidation in redox reactions, where one species gains electrons while another loses them. Understanding reduction is essential in fields like electrochemistry, biochemistry, and industrial processes involving electron transfer.
Definition of Oxidation
Oxidation is a chemical process involving the loss of electrons by a molecule, atom, or ion, often accompanied by the gain of oxygen or the loss of hydrogen. This electron loss results in an increase in the oxidation state, which is a key characteristic of oxidation reactions in both organic and inorganic chemistry. Understanding oxidation is crucial for analyzing redox reactions, where oxidation always occurs simultaneously with reduction.
Key Differences Between Reduction and Oxidation
Reduction involves the gain of electrons, decreasing the oxidation state of an atom, while oxidation is characterized by the loss of electrons and an increase in oxidation state. In redox reactions, reduction and oxidation always occur simultaneously, with one species being reduced as another is oxidized. Key differences also include the roles in energy transfer, where oxidation often releases energy and reduction consumes energy molecules like NADH or FADH2 in cellular respiration.
Common Examples in Everyday Life
Reduction involves the gain of electrons, while oxidation is the loss of electrons, both key processes in redox reactions. Common examples include the rusting of iron, where iron oxidizes by losing electrons to oxygen, and the browning of an apple, which occurs as the fruit's enzymes oxidize phenolic compounds. In batteries, oxidation reactions at the anode release electrons, and reduction reactions at the cathode accept electrons, generating electrical energy used daily.
Electron Transfer: The Core Principle
Reduction involves the gain of electrons by an atom or molecule, while oxidation is defined by the loss of electrons. Electron transfer is the fundamental mechanism driving redox reactions, where one species undergoes oxidation and another undergoes reduction simultaneously. This electron exchange is essential in processes such as cellular respiration, corrosion, and energy production in electrochemical cells.
Role of Oxidizing and Reducing Agents
Oxidizing agents facilitate oxidation by accepting electrons, causing other substances to lose electrons, while reducing agents donate electrons, causing other substances to undergo reduction. Common oxidizing agents include oxygen, chlorine, and potassium permanganate, whereas typical reducing agents consist of hydrogen, carbon, and metals like zinc. The interplay between these agents drives redox reactions fundamental to processes such as cellular respiration, corrosion, and industrial synthesis.
Applications in Industry and Biology
Reduction and oxidation (redox) reactions are fundamental to industrial processes such as metal extraction, corrosion prevention, and energy storage in batteries. In biological systems, redox reactions drive cellular respiration and photosynthesis, enabling energy conversion and metabolic functions. Understanding these processes enhances innovation in biotechnology, environmental monitoring, and renewable energy development.
Tips to Remember Reduction vs Oxidation
Reduction involves the gain of electrons and a decrease in oxidation state, while oxidation involves the loss of electrons and an increase in oxidation state. A useful tip to remember is the acronym OIL RIG: Oxidation Is Loss, Reduction Is Gain (of electrons). Another way to differentiate them is by focusing on oxygen and hydrogen changes--oxidation generally involves gaining oxygen or losing hydrogen, whereas reduction involves losing oxygen or gaining hydrogen.
Summary: Importance in Chemistry
Reduction and oxidation reactions, collectively known as redox processes, are fundamental to chemical transformations and energy transfer in both biological and industrial systems. These reactions involve the transfer of electrons, where oxidation refers to the loss of electrons and reduction to the gain of electrons, driving processes such as respiration, photosynthesis, and corrosion. Understanding redox mechanisms is crucial for developing batteries, catalysts, and environmental technologies that rely on electron exchange.
Reduction Infographic
 libterm.com
libterm.com