Allotropes are different structural forms of the same element, exhibiting distinct physical and chemical properties due to variations in atomic arrangement. Common examples include carbon's allotropes, such as diamond, graphite, and graphene, each with unique applications in industry and technology. Discover how understanding allotropes can enhance your knowledge of material science and their practical uses in the rest of this article.
Table of Comparison
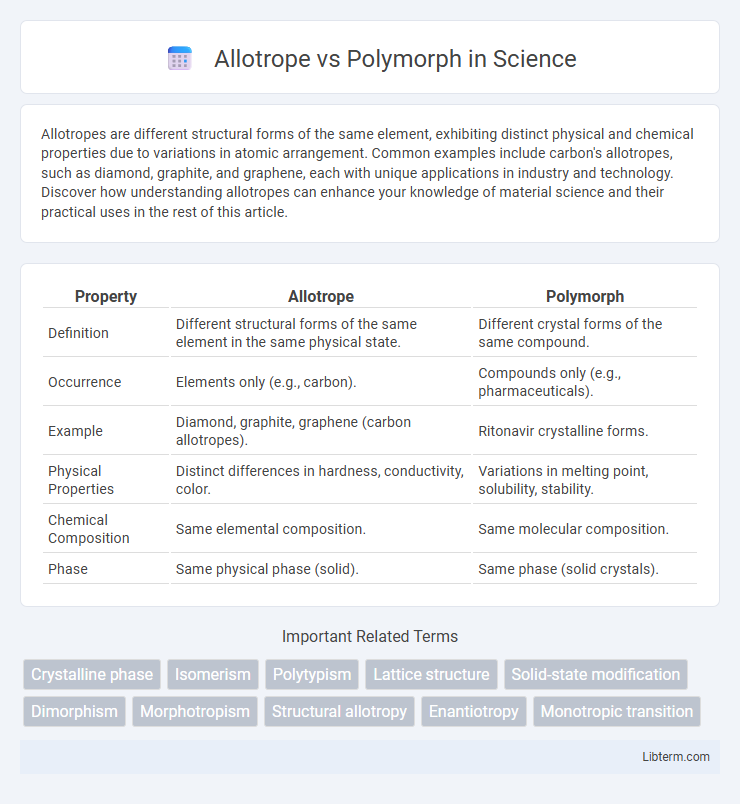
| Property | Allotrope | Polymorph |
|---|---|---|
| Definition | Different structural forms of the same element in the same physical state. | Different crystal forms of the same compound. |
| Occurrence | Elements only (e.g., carbon). | Compounds only (e.g., pharmaceuticals). |
| Example | Diamond, graphite, graphene (carbon allotropes). | Ritonavir crystalline forms. |
| Physical Properties | Distinct differences in hardness, conductivity, color. | Variations in melting point, solubility, stability. |
| Chemical Composition | Same elemental composition. | Same molecular composition. |
| Phase | Same physical phase (solid). | Same phase (solid crystals). |
Introduction to Allotrope and Polymorph
Allotropes are different structural forms of the same element, where atoms are bonded together in distinct arrangements, such as graphite and diamond for carbon, leading to varied physical and chemical properties. Polymorphs refer to different crystalline forms of the same compound, often seen in pharmaceuticals, where the spatial arrangement of molecules affects solubility and stability. Understanding allotropy and polymorphism is crucial in materials science and drug development for optimizing performance and functionality.
Defining Allotrope: Meaning and Examples
An allotrope is a distinct form of an element in the same physical state, characterized by different atomic arrangements or molecular structures, resulting in varied physical and chemical properties. Common examples include carbon allotropes such as diamond, graphite, and graphene, each exhibiting unique hardness, conductivity, and bonding patterns. Understanding allotropes is essential for differentiating them from polymorphs, which refer to different crystal structures of compounds rather than elemental forms.
Understanding Polymorph: Meaning and Examples
Polymorph refers to a substance that can exist in more than one crystalline form, each with distinct molecular arrangements and physical properties. Understanding polymorphs is crucial in pharmaceuticals, as different polymorphic forms of a drug can affect solubility, stability, and bioavailability, exemplified by the polymorphs of carbamazepine or ritonavir. Unlike allotropes, which are variations of an element in different structural forms, polymorphs pertain to compounds, emphasizing their diverse crystal structures rather than elemental states.
Key Differences Between Allotrope and Polymorph
Allotropes are different structural forms of the same element in the same physical state, such as carbon existing as diamond, graphite, or graphene, each with unique atomic arrangements and properties. Polymorphs refer to distinct crystalline forms of the same compound, primarily observed in pharmaceuticals, where variations in molecular packing affect solubility, stability, and bioavailability. The key difference lies in allotropes being elemental modifications with different bonding structures, while polymorphs are molecular compounds with varied crystal lattice arrangements.
Chemical and Physical Properties Comparison
Allotropes and polymorphs differ primarily in their elemental composition and molecular arrangement, with allotropes being different forms of the same element (e.g., carbon as diamond or graphite) and polymorphs referring to various crystal structures of the same compound (e.g., calcium carbonate as calcite or aragonite). Chemically, allotropes exhibit distinct bonding patterns which result in significant variations in reactivity and electronic properties, whereas polymorphs maintain consistent chemical composition but differ in lattice energy and intermolecular forces. Physically, allotropes often display stark contrasts such as hardness and electrical conductivity, while polymorphs show variation in melting points, solubility, and density due to their diverse crystal packing arrangements.
Allotropes in Elemental Substances
Allotropes are different structural forms of the same elemental substance, exhibiting distinct physical and chemical properties due to variations in atomic arrangement. Common examples include carbon allotropes such as diamond, graphite, and graphene, each with unique bonding and characteristics like hardness and electrical conductivity. Polymorphs, by contrast, refer to variations in crystalline structure typically found in compounds rather than pure elements.
Polymorphs in Compounds and Crystals
Polymorphs in compounds and crystals refer to different structural arrangements of the same chemical substance, exhibiting distinct physical and chemical properties despite identical molecular composition. Polymorphism significantly affects the solubility, stability, and bioavailability of pharmaceutical compounds, making it a critical consideration in drug development and formulation. Unlike allotropes, which occur in elemental substances such as carbon or sulfur, polymorphs are primarily observed in complex molecular crystals and solid-state compounds.
Industrial and Scientific Importance
Allotropes and polymorphs exhibit distinct atomic arrangements that critically influence material properties utilized in industrial applications, such as electronics, pharmaceuticals, and catalysis. Allotropes, like graphite and diamond forms of carbon, differ in elemental structure and impart unique mechanical and electrical characteristics, while polymorphs arise from variations in crystal structure of the same compound, affecting solubility and stability vital for drug formulation and manufacturing. Understanding these structural differences enables optimization of performance, durability, and bioavailability in scientific research and commercial products.
Real-world Applications and Case Studies
Allotropes and polymorphs differ in atomic arrangement and molecular structure, influencing their real-world applications across industries. Carbon allotropes like diamond excel in cutting tools due to hardness, while graphite's conductivity suits batteries and lubricants. Polymorphs of pharmaceuticals, such as ritonavir, impact drug solubility and efficacy, underscoring their critical role in medicine formulation and patent strategies.
Summary and Conclusion
Allotropes are different structural forms of the same element existing in the same physical state, such as carbon's diamond and graphite, while polymorphs are different crystalline forms of a compound or element. Allotropes exhibit distinct physical and chemical properties due to variations in atomic bonding and arrangement, whereas polymorphs differ primarily in crystal lattice structure, affecting properties like solubility and stability. Understanding the differences between allotropes and polymorphs is crucial for applications in materials science, pharmaceuticals, and chemistry, as these variations impact functionality and performance.
Allotrope Infographic
 libterm.com
libterm.com