Gasification transforms carbon-rich materials into synthetic gas through high-temperature reactions with limited oxygen, offering a cleaner alternative to traditional combustion processes. This method efficiently converts biomass, coal, and waste into energy, reducing emissions and maximizing fuel utilization. Discover how gasification can enhance your energy strategy by reading the full article.
Table of Comparison
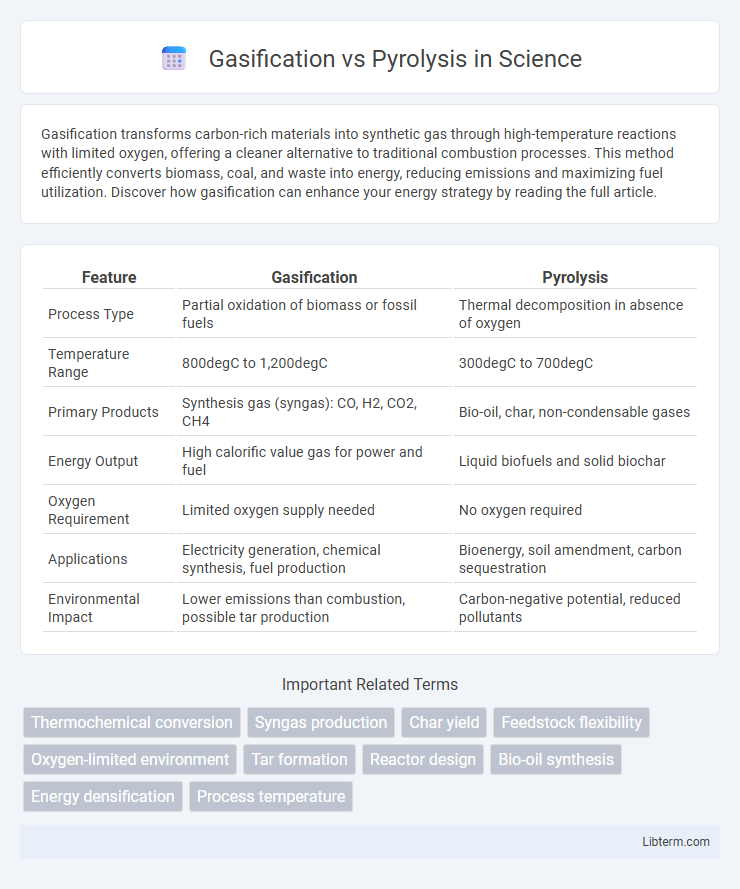
| Feature | Gasification | Pyrolysis |
|---|---|---|
| Process Type | Partial oxidation of biomass or fossil fuels | Thermal decomposition in absence of oxygen |
| Temperature Range | 800degC to 1,200degC | 300degC to 700degC |
| Primary Products | Synthesis gas (syngas): CO, H2, CO2, CH4 | Bio-oil, char, non-condensable gases |
| Energy Output | High calorific value gas for power and fuel | Liquid biofuels and solid biochar |
| Oxygen Requirement | Limited oxygen supply needed | No oxygen required |
| Applications | Electricity generation, chemical synthesis, fuel production | Bioenergy, soil amendment, carbon sequestration |
| Environmental Impact | Lower emissions than combustion, possible tar production | Carbon-negative potential, reduced pollutants |
Introduction to Gasification and Pyrolysis
Gasification converts organic materials into syngas by partial oxidation at high temperatures, enabling efficient energy production from biomass and waste. Pyrolysis thermally decomposes biomass in an oxygen-free environment, generating bio-oil, syngas, and biochar suitable for renewable fuels and soil enhancement. Both processes play key roles in sustainable waste-to-energy technologies, optimizing carbon conversion and resource recovery.
Basic Principles of Gasification
Gasification is a thermochemical process that converts carbonaceous materials into syngas, primarily composed of hydrogen, carbon monoxide, and methane, through partial oxidation at high temperatures above 700degC in a controlled oxygen environment. Unlike pyrolysis, which decomposes organic material in the absence of oxygen to produce bio-oil and char, gasification uses limited oxygen or steam to facilitate chemical reactions that break down feedstock into combustible gases. This makes gasification an efficient technology for energy generation and chemical synthesis from biomass, coal, and waste feedstocks.
Fundamentals of Pyrolysis
Pyrolysis is a thermochemical decomposition process occurring in the absence of oxygen, breaking down organic materials into biochar, bio-oil, and syngas at temperatures typically between 400-600degC. This process involves complex chemical reactions such as depolymerization, fragmentation, and volatilization, enabling the conversion of biomass into valuable energy-rich products. Unlike gasification, which operates at higher temperatures with limited oxygen to produce predominantly syngas, pyrolysis emphasizes the production of liquid bio-oil and solid char, making it fundamental for biofuel and biochemical applications.
Key Differences Between Gasification and Pyrolysis
Gasification converts organic materials into syngas, primarily consisting of carbon monoxide, hydrogen, and carbon dioxide, by reacting at high temperatures with limited oxygen, while pyrolysis thermally decomposes biomass in the absence of oxygen to produce bio-oil, char, and syngas. Gasification operates at higher temperatures (700-1500degC) and involves partial oxidation, enhancing energy yield from feedstocks like coal, biomass, and waste. Pyrolysis favors lower temperatures (300-700degC), emphasizes chemical breakdown, and generates valuable liquid fuels and charcoal, suited for applications in renewable energy and carbon sequestration.
Feedstock Requirements for Each Process
Gasification requires feedstocks with low moisture content, typically below 20%, and a homogeneous composition to ensure efficient conversion into syngas, favoring materials like dry biomass, coal, or municipal solid waste. Pyrolysis can handle a broader range of feedstocks including higher moisture biomass, such as wood chips, agricultural residues, and plastics, due to its thermal decomposition in an oxygen-free environment. Feedstock particle size and uniformity are critical in both processes to optimize reaction rates and product yields, with gasification generally demanding finer and drier materials compared to pyrolysis.
Energy Outputs and Byproducts
Gasification produces syngas composed mainly of hydrogen, carbon monoxide, and methane, offering higher energy content for electricity and fuel synthesis, while generating smaller amounts of tar and char byproducts. Pyrolysis primarily yields bio-oil, syngas with lower calorific value, and biochar, which can be used as soil amendments but typically results in lower overall energy output compared to gasification. Energy efficiency in gasification generally ranges from 60-75%, whereas pyrolysis systems often operate around 50-60%, influenced by feedstock and process conditions.
Environmental Impacts and Emissions
Gasification produces syngas with lower tar and particulate emissions compared to pyrolysis, resulting in reduced air pollutants when properly managed. Pyrolysis generates bio-oil and char with relatively higher volatile organic compounds (VOCs) emissions but offers greater potential for carbon sequestration through biochar application in soils. Both processes help reduce greenhouse gas emissions by converting biomass into energy or valuable products, yet gasification typically achieves higher overall efficiency and lower environmental impact due to its partial oxidation mechanism.
Industrial Applications and Case Studies
Gasification converts carbon-rich materials into syngas using limited oxygen, enabling efficient energy production in steel manufacturing and power plants, demonstrated by successful projects like the Clearwater Gasification Plant. Pyrolysis thermally decomposes organic matter in the absence of oxygen, producing bio-oil, char, and gases, widely applied in waste management and biofuel production, with case studies such as the Anellotech Biochemical Plant showcasing sustainable chemical feedstock generation. Industrial applications favor gasification for large-scale energy recovery, while pyrolysis offers versatility in feedstock processing and value-added products.
Economic Considerations and Scalability
Gasification generally offers higher energy efficiency and commercial scalability, making it more suitable for large-scale waste-to-energy projects with significant capital investment and operational costs. Pyrolysis tends to have lower initial setup costs and better adaptability for small to medium-scale operations but may face economic challenges due to inconsistent feedstock quality and limited market demand for byproducts. Economic feasibility for both technologies heavily depends on feedstock availability, product market values, and regional regulatory incentives.
Future Trends in Thermochemical Conversion Technologies
Gasification and pyrolysis are advancing with a focus on enhancing efficiency, feedstock flexibility, and environmental sustainability. Future trends highlight integration with renewable energy systems, development of catalysts for improved syngas and bio-oil quality, and scaling up modular, decentralized reactors for distributed energy production. Innovations in process monitoring, AI-driven optimization, and carbon capture technologies are expected to drive commercialization and reduce greenhouse gas emissions in thermochemical conversion applications.
Gasification Infographic
 libterm.com
libterm.com