Concentrated solutions contain a high amount of solute relative to the solvent, resulting in a dense mixture with significant chemical properties. Understanding concentration levels is essential for applications ranging from industrial processes to everyday tasks like cooking and cleaning. Explore the full article to learn how concentration affects chemical behavior and practical uses.
Table of Comparison
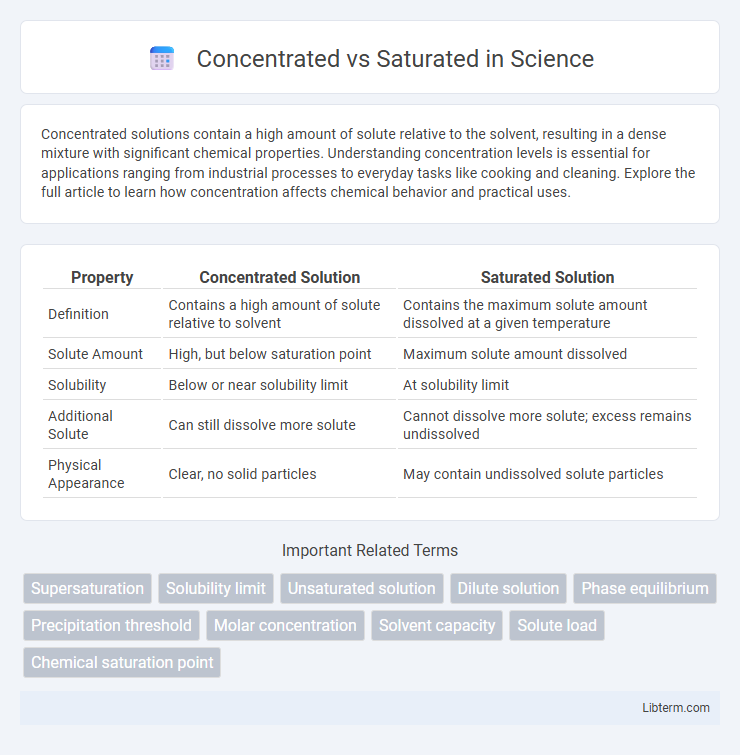
| Property | Concentrated Solution | Saturated Solution |
|---|---|---|
| Definition | Contains a high amount of solute relative to solvent | Contains the maximum solute amount dissolved at a given temperature |
| Solute Amount | High, but below saturation point | Maximum solute amount dissolved |
| Solubility | Below or near solubility limit | At solubility limit |
| Additional Solute | Can still dissolve more solute | Cannot dissolve more solute; excess remains undissolved |
| Physical Appearance | Clear, no solid particles | May contain undissolved solute particles |
Understanding Concentrated Solutions
Concentrated solutions contain a relatively large amount of solute dissolved in a solvent, crucial for applications requiring high solute availability and reactivity. Understanding the concentration involves measuring solute quantity per unit volume, such as molarity (moles per liter), to precisely control chemical reactions and processes. Saturated solutions represent the maximum solute that can dissolve at a given temperature, indicating equilibrium where no more solute dissolves, unlike concentrated solutions where solute quantity is high but not necessarily at the solubility limit.
Defining Saturated Solutions
Saturated solutions contain the maximum amount of solute that can dissolve in a solvent at a given temperature, resulting in an equilibrium where no more solute dissolves. Concentrated solutions have a high amount of solute but have not necessarily reached the solubility limit. Understanding the definition of saturated solutions is essential for distinguishing between simply concentrated mixtures and those that have reached solubility equilibrium.
Key Differences Between Concentrated and Saturated
Concentrated solutions contain a high amount of solute relative to the solvent but are not necessarily at maximum solubility, whereas saturated solutions hold the maximum solute amount a solvent can dissolve at a given temperature. Key differences include that concentrated solutions can still dissolve more solute if conditions change, while saturated solutions are at equilibrium with undissolved solute present. The concentration in concentrated solutions varies widely, but in saturated solutions, the solute concentration is fixed by the solvent's solubility limit.
Factors Affecting Concentration
Concentration depends on factors such as temperature, solute quantity, and solvent volume, with higher temperatures often increasing solute solubility and concentration. Saturated solutions reach a balance where the solvent cannot dissolve more solute at specific conditions, while concentrated solutions contain a high amount of solute but are not necessarily saturated. Understanding the interplay of pressure, mixing, and solute properties further refines how concentration levels are controlled in chemical processes.
Saturation Point Explained
The saturation point refers to the maximum concentration of solute that can dissolve in a solvent at a specific temperature and pressure, beyond which no more solute can dissolve, resulting in a saturated solution. Concentrated solutions contain a high amount of solute but have not yet reached the saturation point, while saturated solutions have reached equilibrium between dissolved solute and undissolved particles. Understanding the saturation point is crucial for applications in chemistry, pharmaceuticals, and environmental science where precise solute concentrations impact reactions and processes.
Examples of Concentrated Solutions
Concentrated solutions contain a high amount of solute dissolved in a solvent, such as a 5 M sodium chloride (NaCl) solution or concentrated sulfuric acid (H2SO4) at 18 M. These solutions often exhibit increased conductivity and viscosity due to the elevated solute concentration. In contrast, saturated solutions reach the maximum solute solubility at a given temperature, like a sugar solution fully dissolved at 25degC where no more sugar can dissolve.
Examples of Saturated Solutions
Saturated solutions occur when a solvent holds the maximum amount of solute at a given temperature, as seen in a sugar-saturated water solution where no more sugar dissolves. Common examples include saltwater at room temperature, where salt crystals remain undissolved despite stirring, and saturated sodium chloride solutions used in chemical labs for precise concentration standards. These examples illustrate how saturation defines equilibrium between dissolved and undissolved solute particles in a solution.
How to Measure Concentration and Saturation
Concentration is measured by quantifying the amount of solute present in a given volume of solution, commonly expressed in units such as molarity (moles per liter) or mass percent. Saturation is determined by assessing whether a solution contains the maximum possible solute at a given temperature, often identified through methods like visual precipitation detection or equilibrium solubility calculations. Precise measurement tools include spectrophotometers for concentration analysis and solubility charts or titration techniques for evaluating saturation levels.
Practical Applications in Industry
Concentrated solutions contain a high amount of solute but are not necessarily at maximum solubility, allowing industries like pharmaceuticals to adjust potency for drug formulations. Saturated solutions hold the maximum solute amount at given conditions, crucial in chemical manufacturing for processes such as crystallization and purity control. Understanding the differences enables precise control over concentration levels in food processing, wastewater treatment, and chemical synthesis.
Choosing the Right Solution for Your Needs
Concentrated solutions contain a higher amount of solute in a given volume compared to saturated solutions, which hold the maximum solute at equilibrium under specific conditions. Choosing the right solution depends on the desired chemical reaction or application, where concentrated solutions provide greater potency and efficiency, while saturated solutions ensure no more solute can dissolve, stabilizing the system. Assessing factors like solubility limits, temperature, and intended use helps determine whether a concentrated or saturated solution best meets your needs.
Concentrated Infographic
 libterm.com
libterm.com