Subatomic particles are the fundamental components that make up atoms, including protons, neutrons, and electrons, each with unique properties influencing matter and energy. Understanding these tiny particles unlocks insights into the nature of the universe and the forces that govern it. Explore the rest of this article to deepen your knowledge of subatomic physics and its fascinating implications.
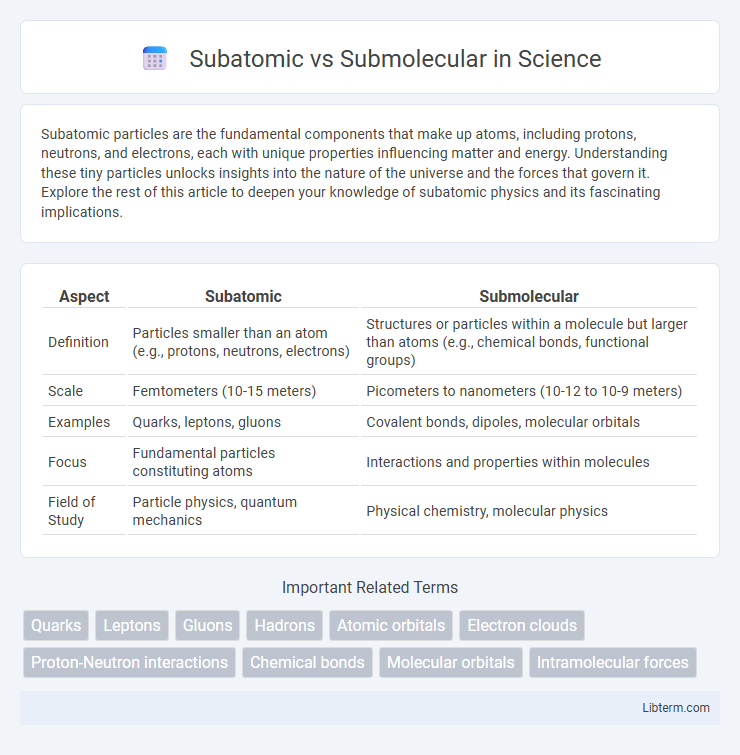
Table of Comparison
| Aspect | Subatomic | Submolecular |
|---|---|---|
| Definition | Particles smaller than an atom (e.g., protons, neutrons, electrons) | Structures or particles within a molecule but larger than atoms (e.g., chemical bonds, functional groups) |
| Scale | Femtometers (10-15 meters) | Picometers to nanometers (10-12 to 10-9 meters) |
| Examples | Quarks, leptons, gluons | Covalent bonds, dipoles, molecular orbitals |
| Focus | Fundamental particles constituting atoms | Interactions and properties within molecules |
| Field of Study | Particle physics, quantum mechanics | Physical chemistry, molecular physics |
Introduction to Subatomic and Submolecular Concepts
Subatomic particles, including protons, neutrons, and electrons, form the fundamental components of atoms, while submolecular structures consist of groups of atoms bonded together within a molecule. The study of subatomic particles explores quantum mechanics and particle physics to understand matter at its most basic level, whereas submolecular concepts focus on molecular geometry, bonding interactions, and chemical behavior. Understanding the distinction between subatomic and submolecular scales is essential for fields such as nuclear physics, molecular chemistry, and materials science.
Defining Subatomic Particles
Subatomic particles are the fundamental components of atoms, including protons, neutrons, and electrons, which define the atom's structure and properties. These particles are smaller than atoms and are crucial in nuclear reactions and quantum physics. Submolecular structures refer to groups of atoms within molecules, focusing on chemical bonds rather than the elementary particles that subatomic describes.
Understanding Submolecular Structures
Submolecular structures refer to the arrangement and interactions of particles smaller than molecules, including atoms, ions, and subunits such as functional groups, which determine the chemical and physical properties of substances. Understanding submolecular structures involves studying the spatial configuration and bonding patterns within molecules, essential for fields like biochemistry and nanotechnology. While subatomic particles like protons, neutrons, and electrons form atoms, submolecular analysis focuses on how these atoms combine and interact to create complex molecular architectures.
Key Differences: Subatomic vs Submolecular
Subatomic particles refer to constituents smaller than an atom, such as protons, neutrons, and electrons that define atomic structure, while submolecular components involve parts smaller than a molecule but larger than individual atoms, including functional groups and molecular fragments. The key difference lies in scale and complexity: subatomic focuses on fundamental particles and forces within an atom, whereas submolecular pertains to arrangements and interactions of atoms within molecules affecting chemical properties. Understanding these distinctions is crucial for fields like particle physics and molecular chemistry, where particle behavior and molecular configuration drive different scientific models and applications.
Role in Quantum Physics and Chemistry
Subatomic particles, such as protons, neutrons, and electrons, are fundamental components of atoms and play a crucial role in quantum physics by exhibiting wave-particle duality and quantized energy states. Submolecular structures involve the arrangement and interactions of atoms within molecules, influencing chemical bonding and reactions through quantum mechanical principles like electron orbitals and molecular orbitals. Understanding both subatomic and submolecular levels is essential for modeling atomic interactions, predicting chemical behavior, and developing technologies in quantum chemistry and quantum computing.
Examples of Subatomic Particles
Subatomic particles include protons, neutrons, and electrons, which are fundamental constituents of atoms. In contrast, submolecular entities refer to components smaller than molecules but not fundamental particles, such as atoms within a molecule or molecular fragments. Protons and neutrons themselves are made of quarks, representing a deeper level of subatomic structure.
Examples of Submolecular Structures
Submolecular structures refer to the distinct configurations within molecules, such as functional groups like hydroxyl (-OH) and carboxyl (-COOH), which influence chemical behavior and reactivity. In contrast, subatomic particles--including protons, neutrons, and electrons--constitute atoms themselves and serve as the fundamental basis for molecular formation. Examples of submolecular structures include peptide bonds in proteins and phosphate groups in nucleic acids, illustrating the complexity beyond atomic composition.
Impact on Modern Scientific Research
Subatomic particles such as protons, neutrons, and electrons form the foundation of atomic structure, while submolecular interactions involve the forces governing molecular behavior and chemical reactions. Advances in subatomic physics have propelled quantum mechanics and particle accelerator research, critically enhancing our understanding of fundamental forces and particle collisions. Research on submolecular phenomena drives innovations in materials science, drug design, and nanotechnology by elucidating molecular bonding and interaction mechanisms at atomic scales.
Technological Applications of Subatomic and Submolecular Science
Subatomic particles such as electrons, protons, and neutrons play a crucial role in the development of quantum computing, where their quantum states enable unprecedented computational power. Submolecular science, focusing on intermediates between molecules and atoms, drives advancements in nanotechnology and molecular electronics by manipulating molecular structures for enhanced material properties. Innovations in medical imaging techniques like MRI also rely on subatomic particle behavior to improve diagnostic precision and treatment efficacy.
Future Perspectives in Subatomic and Submolecular Studies
Emerging advancements in particle accelerators and quantum computing are set to revolutionize subatomic research by enabling unprecedented precision in probing fundamental particles like quarks and leptons. Submolecular studies are expected to benefit from developments in ultra-fast spectroscopy and nanoscale imaging, offering deeper insights into molecular interactions and quantum behavior beyond atomic nuclei. These technological innovations will drive breakthroughs in materials science, quantum chemistry, and particle physics, shaping future explorations of matter at the smallest scales.
Subatomic Infographic
 libterm.com
libterm.com