Polymerization is a chemical process that combines small molecules called monomers into large, chain-like structures known as polymers, which are essential in producing plastics, resins, and synthetic fibers. Understanding the types of polymerization, such as addition and condensation, is crucial for optimizing material properties for various applications. Explore the rest of the article to discover how polymerization impacts industries and your daily life.
Table of Comparison
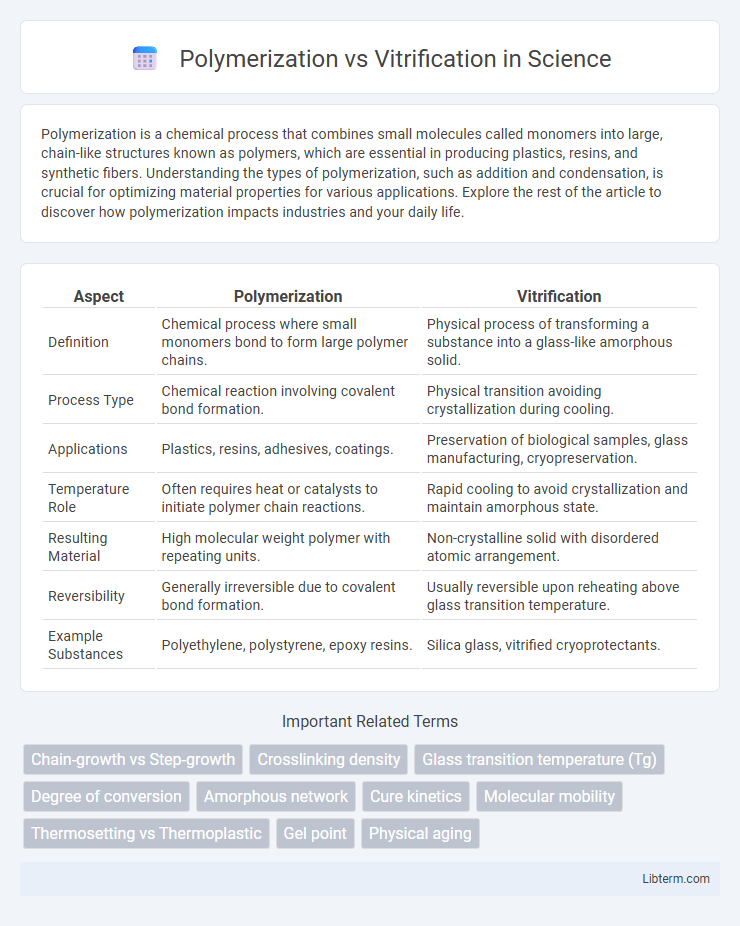
| Aspect | Polymerization | Vitrification |
|---|---|---|
| Definition | Chemical process where small monomers bond to form large polymer chains. | Physical process of transforming a substance into a glass-like amorphous solid. |
| Process Type | Chemical reaction involving covalent bond formation. | Physical transition avoiding crystallization during cooling. |
| Applications | Plastics, resins, adhesives, coatings. | Preservation of biological samples, glass manufacturing, cryopreservation. |
| Temperature Role | Often requires heat or catalysts to initiate polymer chain reactions. | Rapid cooling to avoid crystallization and maintain amorphous state. |
| Resulting Material | High molecular weight polymer with repeating units. | Non-crystalline solid with disordered atomic arrangement. |
| Reversibility | Generally irreversible due to covalent bond formation. | Usually reversible upon reheating above glass transition temperature. |
| Example Substances | Polyethylene, polystyrene, epoxy resins. | Silica glass, vitrified cryoprotectants. |
Introduction to Polymerization and Vitrification
Polymerization is a chemical process where monomers link to form long-chain polymers, creating materials with diverse mechanical and chemical properties essential for plastics, adhesives, and coatings. Vitrification refers to the transformation of a substance into a glassy, amorphous solid state without crystallization, commonly used in preserving biological samples and waste immobilization. Both processes involve phase changes but differ fundamentally in mechanism and applications, with polymerization emphasizing molecular bonding and vitrification focusing on structural solidification.
Defining Polymerization: Process and Applications
Polymerization is a chemical process where small molecules called monomers chemically bond to form long-chain polymers with distinct properties. This reaction occurs through mechanisms like addition or condensation, enabling the creation of materials such as plastics, adhesives, and resins. Applications of polymerization span diverse industries, including automotive manufacturing, electronics, and biomedical devices, highlighting its critical role in material science and engineering.
Understanding Vitrification: Mechanisms and Uses
Vitrification is a process where liquids or amorphous materials transform into a glass-like solid without crystallization, achieved by rapid cooling that prevents molecular rearrangement. This technique is widely used in cryopreservation to protect biological samples, such as embryos and tissues, by avoiding ice crystal formation that damages cells. The molecular immobilization during vitrification maintains structural integrity, making it essential for applications in both medical preservation and advanced material sciences.
Key Differences Between Polymerization and Vitrification
Polymerization involves the chemical process of linking monomers into long polymer chains, resulting in a solid or gel-like material, whereas vitrification is the physical transformation of a substance into a glassy, amorphous solid without crystalline structure. The key difference lies in polymerization's reliance on chemical bond formation to create macromolecules, while vitrification depends on rapid cooling or dehydration to bypass crystallization and achieve a rigid, glassy state. Polymerization typically produces materials with defined molecular architectures, whereas vitrification results in disordered, non-crystalline solids commonly used in cryopreservation and material science.
Chemical and Physical Changes in Both Processes
Polymerization involves chemical changes where monomers chemically bond to form long polymer chains, creating new substances with distinct molecular structures. Vitrification primarily entails physical changes, as it transforms amorphous materials into a glassy, non-crystalline solid without altering the chemical composition. The chemical transformation in polymerization contrasts with vitrification's physical state shift, highlighting differences in molecular rearrangement and bonding under thermal or catalytic influence.
Industrial Applications: Polymerization vs. Vitrification
Polymerization is widely used in industries such as plastics, adhesives, and coatings to create long-chain polymers with customizable properties, enabling mass production of materials like polyethylene and epoxy resins. Vitrification finds crucial industrial applications in waste management and electronics, where molten glass immobilizes hazardous materials or forms glassy matrices for semiconductor components. The choice between polymerization and vitrification depends on desired end-use properties, such as flexibility and chemical resistance in polymers versus thermal stability and inertness in vitrified products.
Advantages and Limitations of Polymerization
Polymerization offers advantages such as increased material strength, flexibility, and ease of processing, making it ideal for manufacturing plastics, coatings, and adhesives with customizable properties. Limitations include sensitivity to temperature and pressure conditions, potential for incomplete reactions leading to defects, and environmental concerns due to the use of toxic monomers and generation of hazardous waste. Despite these drawbacks, polymerization remains a versatile method favored in industries requiring lightweight, durable, and cost-effective materials.
Benefits and Drawbacks of Vitrification
Vitrification offers the benefit of transforming materials into a stable, glass-like state that is highly resistant to chemical and thermal degradation, making it ideal for long-term containment of hazardous substances such as nuclear waste. The process reduces volume and immobilizes contaminants, minimizing environmental impact and enhancing safety during storage and disposal. However, vitrification can be energy-intensive and expensive, with potential challenges in scaling up for large waste volumes and managing the brittleness of the final vitrified product.
Real-World Examples and Case Studies
Polymerization, exemplified by the creation of polyethylene in plastic packaging, enhances material flexibility and durability through chain linking of monomers, while vitrification, observed in the immobilization of radioactive waste, transforms substances into stable, glass-like solids by rapid cooling. Case studies in waste management reveal vitrification's success in safely containing hazardous elements, contrasting with polymerization's widespread use in manufacturing for producing resilient polymers such as epoxy resins used in aerospace. These real-world applications underscore polymerization's role in structural material development and vitrification's critical function in environmental safety and long-term containment.
Future Trends in Polymerization and Vitrification Technology
Future trends in polymerization emphasize controlled and sustainable processes, leveraging advanced catalysts and photopolymerization techniques to enhance precision and reduce environmental impact. Vitrification technology is evolving towards improved energy efficiency and real-time monitoring systems to optimize glass formation and durability, especially in waste immobilization and advanced materials. Integration of machine learning and automation is accelerating innovation in both fields, enabling faster development cycles and tailored material properties for specific industrial applications.
Polymerization Infographic
 libterm.com
libterm.com