An isochoric process occurs when the volume of a gas remains constant while its pressure and temperature change. This process is often studied in thermodynamics to understand how pressure varies with temperature under rigid conditions. Explore the detailed properties and applications of isochoric processes in the rest of this article.
Table of Comparison
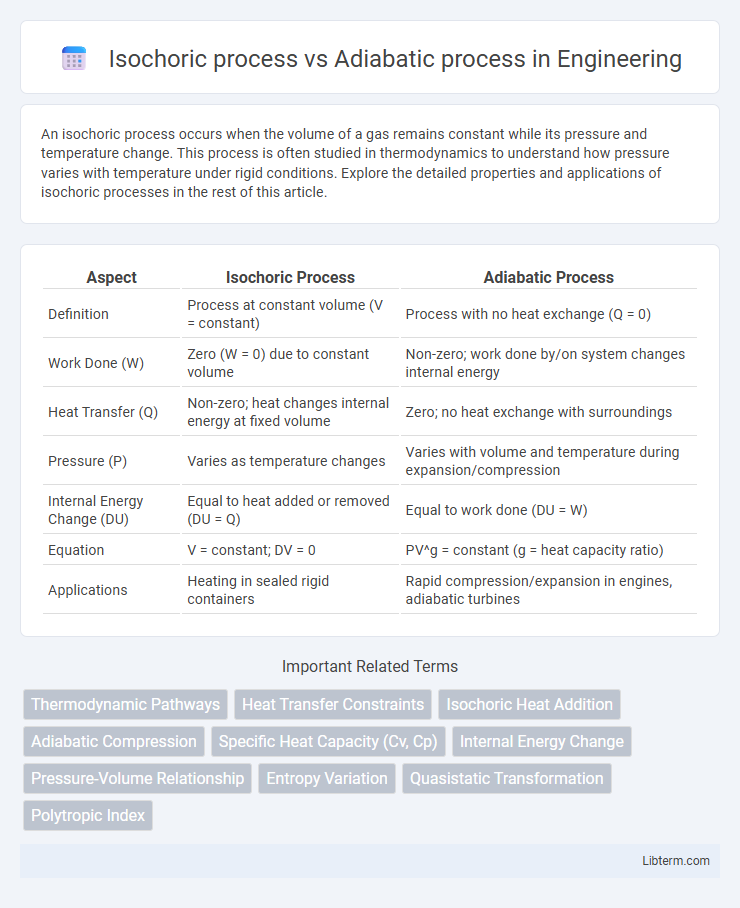
| Aspect | Isochoric Process | Adiabatic Process |
|---|---|---|
| Definition | Process at constant volume (V = constant) | Process with no heat exchange (Q = 0) |
| Work Done (W) | Zero (W = 0) due to constant volume | Non-zero; work done by/on system changes internal energy |
| Heat Transfer (Q) | Non-zero; heat changes internal energy at fixed volume | Zero; no heat exchange with surroundings |
| Pressure (P) | Varies as temperature changes | Varies with volume and temperature during expansion/compression |
| Internal Energy Change (DU) | Equal to heat added or removed (DU = Q) | Equal to work done (DU = W) |
| Equation | V = constant; DV = 0 | PV^g = constant (g = heat capacity ratio) |
| Applications | Heating in sealed rigid containers | Rapid compression/expansion in engines, adiabatic turbines |
Introduction to Isochoric and Adiabatic Processes
Isochoric processes involve thermodynamic changes at constant volume, where no work is done by the system and any heat transfer directly alters the internal energy. Adiabatic processes occur without heat exchange, causing temperature and pressure changes solely due to internal energy variations from work done on or by the system. Both processes play crucial roles in thermodynamics, with isochoric paths characterized by volume constancy and adiabatic paths defined by insulation from heat transfer.
Defining Isochoric Process
An isochoric process is a thermodynamic process in which the volume remains constant, meaning no work is done by the system since volume does not change. During an isochoric process, heat transfer causes a change in internal energy and pressure but the system's volume stays fixed. In contrast, an adiabatic process involves no heat exchange with the surroundings, resulting in changes to both pressure and volume solely due to internal energy transformations.
Defining Adiabatic Process
An adiabatic process is a thermodynamic transformation in which no heat exchange occurs between the system and its surroundings, meaning the system is perfectly insulated. In contrast, an isochoric process occurs at constant volume, involving heat transfer but no work done by expansion or compression. The adiabatic process is characterized by changes in pressure and temperature solely due to internal energy variations, adhering to the first law of thermodynamics without heat transfer (Q=0).
Key Differences Between Isochoric and Adiabatic Processes
The Isochoric process maintains a constant volume, resulting in no work done since volume does not change, whereas the Adiabatic process involves no heat exchange with the surroundings and allows volume to change, performing work on or by the system. In an Isochoric process, pressure and temperature vary with heat transfer, while in an Adiabatic process, pressure and temperature changes occur due to work done without heat transfer. Thermodynamic equations for Isochoric processes use constant volume heat capacity (Cv), whereas Adiabatic processes follow the Poisson relations involving pressure, volume, and temperature.
Thermodynamic Laws Governing Each Process
An isochoric process occurs at constant volume where no work is done, and the first law of thermodynamics directly relates the heat added to the change in internal energy. An adiabatic process features no heat exchange with the surroundings, relying solely on changes in internal energy to perform work, following the first law as well but with Q = 0. Both processes demonstrate conservation of energy, yet differ fundamentally in heat transfer constraints governed by thermodynamic principles.
Pressure, Volume, and Temperature Relationships
In an isochoric process, volume remains constant while pressure is directly proportional to temperature, following Gay-Lussac's law (P/T = constant). In contrast, an adiabatic process involves no heat exchange, causing pressure, volume, and temperature to change according to the relation \(PV^\gamma = \text{constant}\), where \(\gamma\) is the heat capacity ratio. Temperature decreases during expansion and increases during compression in adiabatic processes, unlike the constant volume in isochoric conditions.
Real-World Examples of Isochoric Processes
The isochoric process, characterized by constant volume with pressure and temperature changes, commonly occurs in real-world applications such as the heating of gas in a rigid container or certain industrial pressure vessels where volume remains fixed. Unlike the adiabatic process, which involves no heat exchange and changes in both pressure and volume, isochoric processes are crucial in safety devices like pressure cookers and gas thermometers for precise control of thermodynamic variables. Understanding the practical roles of isochoric processes enables better design and operation of equipment requiring volume constancy under varying thermal conditions.
Real-World Examples of Adiabatic Processes
Adiabatic processes occur frequently in natural and engineered systems, such as the rapid expansion of gas in a piston engine where no heat is exchanged with the environment, preserving internal energy changes through work alone. Atmospheric phenomena like rising air parcels undergoing adiabatic cooling and the operation of refrigeration cycles also exemplify real-world adiabatic processes. In contrast, isochoric processes, characterized by constant volume, are seen in rigid containers where pressure and temperature change without volume variation, such as in fixed-volume combustion chambers during ignition.
Applications in Engineering and Physics
Isochoric processes, where volume remains constant, are crucial in internal combustion engines for modeling combustion chamber pressure changes without volume change. Adiabatic processes, characterized by no heat exchange, are fundamental in thermodynamics for analyzing turbine, compressor operations, and atmospheric thermodynamics. Both processes underpin efficient energy transformations in engineering systems and predictive models in physical sciences.
Summary: Isochoric vs Adiabatic Processes
Isochoric processes occur at constant volume, causing pressure and temperature to change without work done by the system, while adiabatic processes involve no heat exchange, leading to simultaneous changes in pressure, volume, and temperature. In isochoric processes, internal energy changes directly relate to heat transfer since volume remains fixed, whereas adiabatic processes rely on pressure-volume work affecting internal energy without heat flow. These fundamental differences distinguish thermodynamic pathways critical in engine cycles and gas law applications.
Isochoric process Infographic
 libterm.com
libterm.com