Osmotic pressure is the force exerted by a solvent when it moves through a semipermeable membrane from a less concentrated solution to a more concentrated one. This phenomenon plays a crucial role in biological processes and industrial applications by maintaining fluid balance and driving nutrient absorption. Explore the rest of this article to understand how osmotic pressure impacts your everyday life and its practical uses.
Table of Comparison
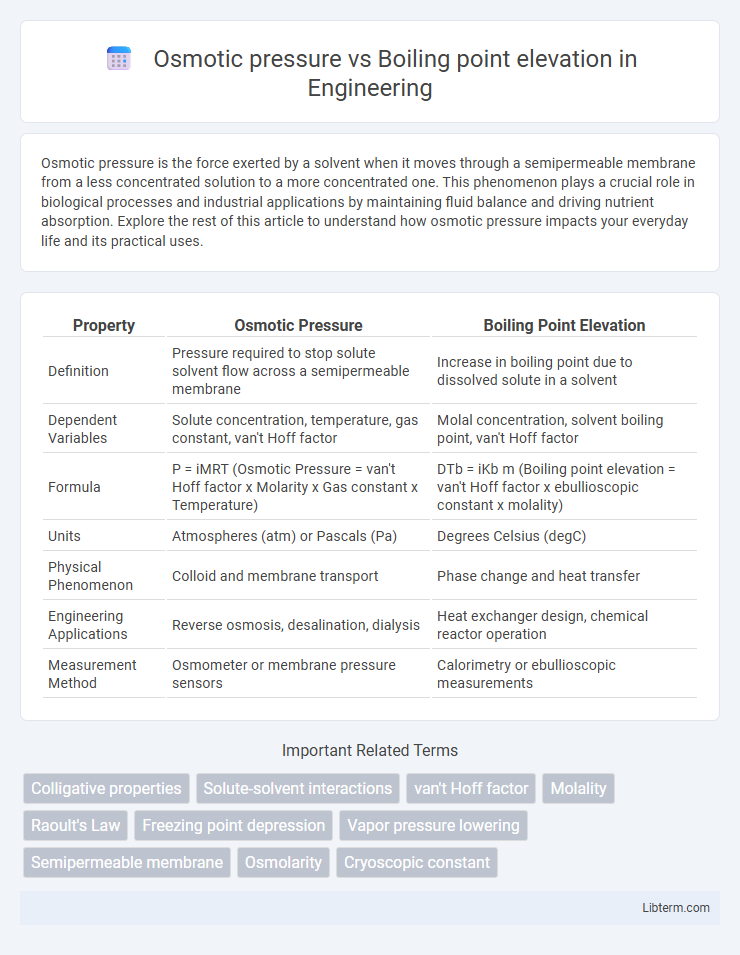
| Property | Osmotic Pressure | Boiling Point Elevation |
|---|---|---|
| Definition | Pressure required to stop solute solvent flow across a semipermeable membrane | Increase in boiling point due to dissolved solute in a solvent |
| Dependent Variables | Solute concentration, temperature, gas constant, van't Hoff factor | Molal concentration, solvent boiling point, van't Hoff factor |
| Formula | P = iMRT (Osmotic Pressure = van't Hoff factor x Molarity x Gas constant x Temperature) | DTb = iKb m (Boiling point elevation = van't Hoff factor x ebullioscopic constant x molality) |
| Units | Atmospheres (atm) or Pascals (Pa) | Degrees Celsius (degC) |
| Physical Phenomenon | Colloid and membrane transport | Phase change and heat transfer |
| Engineering Applications | Reverse osmosis, desalination, dialysis | Heat exchanger design, chemical reactor operation |
| Measurement Method | Osmometer or membrane pressure sensors | Calorimetry or ebullioscopic measurements |
Introduction to Colligative Properties
Colligative properties depend on the number of solute particles in a solvent, not their identity, making osmotic pressure and boiling point elevation intrinsic to solution chemistry. Osmotic pressure measures the force required to stop solvent flow through a semipermeable membrane, directly correlating with solute concentration and temperature. Boiling point elevation occurs when solute particles disrupt vapor pressure, requiring higher temperatures to boil, with magnitude proportional to molal concentration and solvent-specific constants.
Defining Osmotic Pressure
Osmotic pressure is the pressure required to prevent the flow of solvent through a semipermeable membrane from a dilute to a concentrated solution, directly related to solute concentration and temperature. Unlike boiling point elevation, which involves the increase in boiling temperature due to solute presence, osmotic pressure measures the tendency of solvent molecules to move and equalize solute concentration. Both colligative properties depend on solute particle number but describe different physical phenomena influencing solution behavior.
Understanding Boiling Point Elevation
Boiling point elevation occurs when a solute is dissolved in a solvent, causing an increase in the solvent's boiling temperature due to a decrease in vapor pressure. This colligative property depends on the number of solute particles rather than their identity, making it directly proportional to molal concentration and the van't Hoff factor. Understanding boiling point elevation is essential in applications such as antifreeze formulation and determining molecular weights through colligative property measurements.
Key Differences Between Osmotic Pressure and Boiling Point Elevation
Osmotic pressure measures the force required to prevent solvent flow across a semipermeable membrane, directly related to solute concentration and temperature by the formula P = iMRT. Boiling point elevation represents the increase in a solvent's boiling temperature due to solute presence, proportional to the molal concentration and solvent-specific ebullioscopic constant (DTb = Kb*m). While osmotic pressure depends on solvent movement and membrane permeability, boiling point elevation involves vapor pressure lowering and does not require a membrane, highlighting distinct colligative property mechanisms.
The Science Behind Osmotic Pressure
Osmotic pressure arises from the movement of solvent molecules through a semipermeable membrane to balance solute concentrations, driven by differences in chemical potential. It depends on solute concentration, temperature, and the nature of the solute particles, calculated using the van't Hoff equation p = iMRT, where p is osmotic pressure, i the ionization factor, M molarity, R the gas constant, and T temperature in Kelvin. In contrast, boiling point elevation occurs due to solute particles lowering solvent vapor pressure, increasing the temperature required for the liquid to boil, directly related to molal concentration and solvent properties rather than membrane dynamics.
Mechanisms of Boiling Point Elevation
Boiling point elevation occurs when a solute dissolves in a solvent, disrupting the solvent's vapor pressure and requiring higher temperatures to reach boiling. This colligative property depends on the number of solute particles, which interfere with solvent molecule escape, thus increasing the boiling point proportionally to the molal concentration. Unlike osmotic pressure, which relates to solvent movement through a semipermeable membrane, boiling point elevation directly involves vapor pressure lowering and thermal energy changes at the liquid-gas interface.
Factors Influencing Osmotic Pressure and Boiling Point Elevation
Osmotic pressure depends primarily on solute concentration, temperature, and the nature of the solute particles, with higher solute concentrations and temperatures increasing the pressure. Boiling point elevation is influenced by the solute's molality, the solvent's vapor pressure, and the van't Hoff factor, which accounts for solute dissociation in solution. Both properties are colligative, relying on the number of solute particles rather than their identity, but osmotic pressure can be more sensitive to variations in temperature and particle size.
Real-life Applications and Examples
Osmotic pressure is crucial in medical treatments such as intravenous (IV) fluid administration, where isotonic solutions prevent cell damage, while boiling point elevation plays a vital role in food preservation by increasing the boiling temperature to kill microbes effectively. In desalination plants, osmotic pressure aids reverse osmosis systems to purify water by forcing solvent through semipermeable membranes, whereas boiling point elevation is used in antifreeze formulations to prevent engine coolant from boiling under high temperatures. Both phenomena exploit colligative properties of solutions, demonstrating their practical importance in healthcare, environmental management, and industrial processes.
Measurement and Calculation Methods
Osmotic pressure is measured using osmometry techniques that determine solvent flow through a semipermeable membrane, calculated by van't Hoff's equation p = iMRT, where p is osmotic pressure, i the ionization factor, M molarity, R the gas constant, and T temperature in Kelvin. Boiling point elevation is measured by identifying the temperature increase of a solvent's boiling point when a solute is dissolved, calculated with the formula DTb = iKb m, where DTb is the boiling point elevation, i the van't Hoff factor, Kb the ebullioscopic constant, and m the molality of the solution. Both measurements rely on colligative property theories but differ in experimental setup and the physical properties they quantify.
Summary: Osmotic Pressure vs Boiling Point Elevation
Osmotic pressure and boiling point elevation both result from solute presence in a solvent, but osmotic pressure measures the force needed to prevent solvent flow across a semipermeable membrane, while boiling point elevation refers to the increase in temperature required for the solvent to boil. Osmotic pressure depends on solute concentration, temperature, and the gas constant, quantified by the formula p = iMRT, whereas boiling point elevation relies on the molal concentration of the solute and the solvent's ebullioscopic constant, given by DTb = iKbm. These colligative properties highlight different physical phenomena driven by solute particles in solutions, essential for applications in chemical engineering and biological systems.
Osmotic pressure Infographic
 libterm.com
libterm.com