Electroless plating offers a uniform metal coating without using an external electrical source, providing enhanced corrosion resistance and wear protection for complex-shaped surfaces. This chemical reduction process deposits metals like nickel or copper evenly, improving your components' durability and performance. Discover how electroless plating can revolutionize your manufacturing process by reading the full article.
Table of Comparison
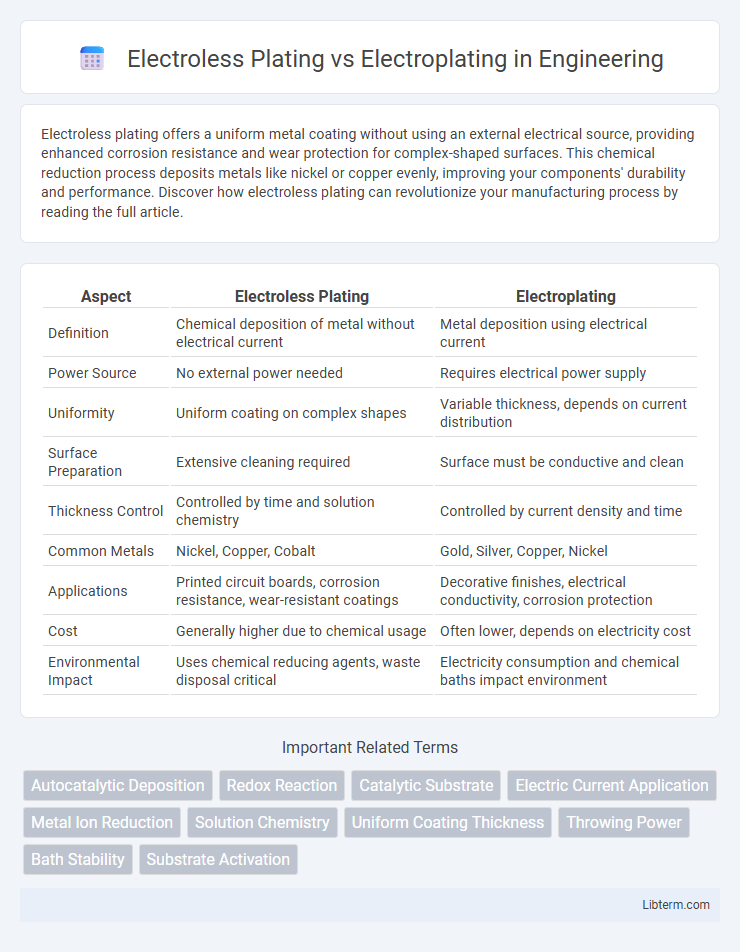
| Aspect | Electroless Plating | Electroplating |
|---|---|---|
| Definition | Chemical deposition of metal without electrical current | Metal deposition using electrical current |
| Power Source | No external power needed | Requires electrical power supply |
| Uniformity | Uniform coating on complex shapes | Variable thickness, depends on current distribution |
| Surface Preparation | Extensive cleaning required | Surface must be conductive and clean |
| Thickness Control | Controlled by time and solution chemistry | Controlled by current density and time |
| Common Metals | Nickel, Copper, Cobalt | Gold, Silver, Copper, Nickel |
| Applications | Printed circuit boards, corrosion resistance, wear-resistant coatings | Decorative finishes, electrical conductivity, corrosion protection |
| Cost | Generally higher due to chemical usage | Often lower, depends on electricity cost |
| Environmental Impact | Uses chemical reducing agents, waste disposal critical | Electricity consumption and chemical baths impact environment |
Introduction to Metal Plating Technologies
Electroless plating and electroplating are two fundamental metal plating technologies used to enhance surface properties such as corrosion resistance, wear resistance, and electrical conductivity. Electroless plating involves a chemical reduction process without electrical current, enabling uniform coatings on complex shapes and non-conductive substrates. Electroplating requires an external power source to deposit metal ions onto a conductive surface, offering precise control over coating thickness and composition.
What is Electroless Plating?
Electroless plating is a chemical deposition process that deposits a metal coating on a substrate without using an external electric current, relying instead on an autocatalytic chemical reaction. This method provides uniform coating thickness even on complex geometries and non-conductive surfaces, making it ideal for applications requiring precise corrosion resistance and wear protection. Common metals used in electroless plating include nickel, copper, and gold, chosen for their enhanced hardness and excellent adhesion properties.
What is Electroplating?
Electroplating is a metal coating process that uses an electric current to reduce dissolved metal cations onto a conductive substrate, creating a uniform, durable layer. This technique enhances corrosion resistance, wear resistance, and aesthetic appeal by depositing metals like nickel, chromium, or gold onto surfaces. Electroplating requires a power source, electrolyte solution, and electrodes, differentiating it from electroless plating, which relies on a chemical reduction process without external current.
Electroless Plating: Process and Mechanism
Electroless plating is an autocatalytic chemical process that deposits a metal layer on a substrate without using an external electrical current, differentiating it from electroplating. The mechanism involves a controlled redox reaction where metal ions in an aqueous solution are reduced to solid metal by a chemical reducing agent, commonly sodium hypophosphite or sodium borohydride. This uniform deposition method ensures consistent thickness, excellent corrosion resistance, and the ability to coat complex geometries, making it ideal for applications requiring precision and durability.
Electroplating: Process and Mechanism
Electroplating involves the deposition of a metal coating on a substrate through an electrochemical process, where an electric current reduces metal cations from a solution onto the workpiece acting as the cathode. The mechanism relies on the electrolytic cell setup, typically comprising an anode of the plating metal, a cathode substrate, and an electrolyte containing metal salts and additives that influence deposit quality and adhesion. Electroplating offers precise control over coating thickness, thickness uniformity, and surface properties, making it ideal for improving corrosion resistance, wear resistance, and aesthetic appeal in industries such as automotive, electronics, and jewelry manufacturing.
Key Differences Between Electroless Plating and Electroplating
Electroless plating uses a chemical reduction process to deposit a metal coating uniformly on a substrate without the need for an electric current, making it ideal for complex shapes and internal surfaces. Electroplating requires an external electrical source to reduce metal ions onto the substrate, offering faster deposition rates but limited to conductive surfaces. The key differences lie in their mechanisms, uniformity of coating, and application suitability, with electroless plating providing consistent thickness and electroplating offering higher control over thickness variability.
Advantages of Electroless Plating
Electroless plating offers uniform coating thickness on complex geometries without requiring electrical current, enabling superior corrosion resistance and enhanced wear properties on non-conductive substrates. It provides excellent adhesion and consistent layer deposition, eliminating the need for masking and reducing production costs. This method ensures improved hardness and chemical stability, making it ideal for precision components in automotive, aerospace, and electronics industries.
Advantages of Electroplating
Electroplating offers superior coating uniformity and thickness control compared to electroless plating, enabling precise deposition on complex geometries. It provides enhanced adhesion strength and improved corrosion resistance due to the strong electrochemical bonding of the metal layer. High production efficiency and cost-effectiveness make electroplating ideal for large-scale industrial applications requiring consistent, high-quality metal finishes.
Applications of Electroless Plating vs Electroplating
Electroless plating is widely applied in industries requiring uniform coatings on complex geometries, such as aerospace components, electronics, and automotive parts, where precise thickness control and corrosion resistance are critical. Electroplating finds extensive use in decorative finishes, electrical connectors, and hardware manufacturing due to its cost-effectiveness and ability to deposit thick metal layers quickly. While electroless plating excels in non-conductive substrate coating and fine detail applications, electroplating remains dominant for large-scale, conductive metal surface treatments.
Choosing the Right Plating Method: Factors to Consider
Selecting the appropriate plating method requires evaluating factors such as substrate material compatibility, desired coating uniformity, and application environment. Electroless plating offers consistent thickness and excellent corrosion resistance on complex geometries, while electroplating provides faster deposition rates and is cost-effective for conductive surfaces. Consider parameters like component shape, production scale, and performance requirements to optimize coating quality and durability.
Electroless Plating Infographic
 libterm.com
libterm.com