Osmotic pressure is the force exerted by a solvent as it moves through a semipermeable membrane to balance solute concentrations on both sides. This phenomenon plays a crucial role in biological systems, influencing cellular hydration and nutrient absorption. Explore the rest of the article to understand how osmotic pressure impacts your daily life and scientific applications.
Table of Comparison
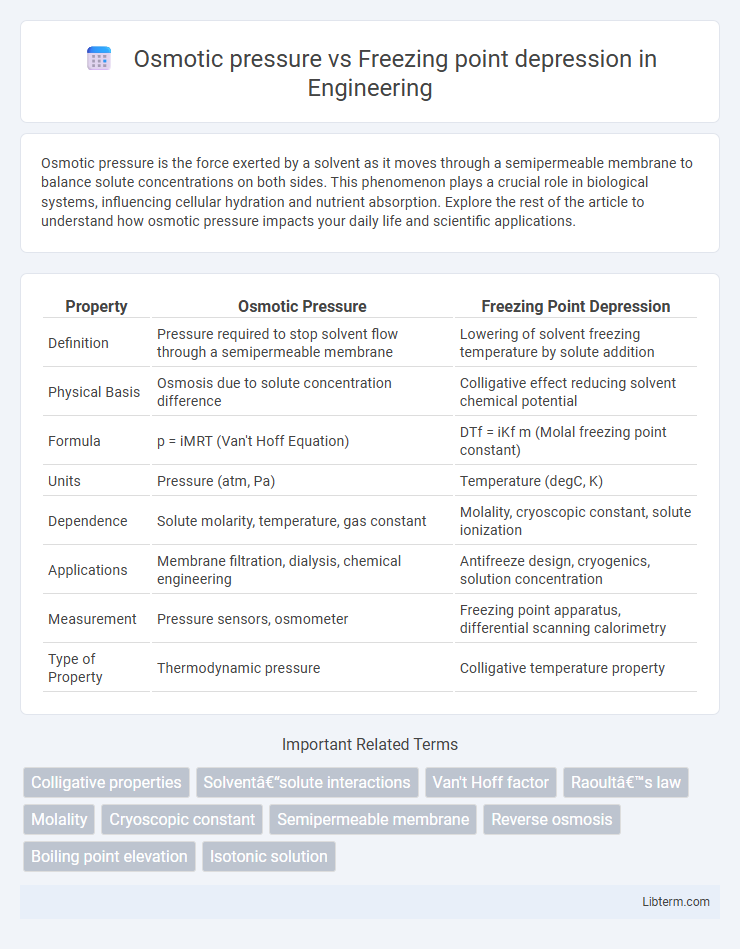
| Property | Osmotic Pressure | Freezing Point Depression |
|---|---|---|
| Definition | Pressure required to stop solvent flow through a semipermeable membrane | Lowering of solvent freezing temperature by solute addition |
| Physical Basis | Osmosis due to solute concentration difference | Colligative effect reducing solvent chemical potential |
| Formula | p = iMRT (Van't Hoff Equation) | DTf = iKf m (Molal freezing point constant) |
| Units | Pressure (atm, Pa) | Temperature (degC, K) |
| Dependence | Solute molarity, temperature, gas constant | Molality, cryoscopic constant, solute ionization |
| Applications | Membrane filtration, dialysis, chemical engineering | Antifreeze design, cryogenics, solution concentration |
| Measurement | Pressure sensors, osmometer | Freezing point apparatus, differential scanning calorimetry |
| Type of Property | Thermodynamic pressure | Colligative temperature property |
Introduction to Colligative Properties
Colligative properties depend solely on the number of solute particles in a solvent, not their identity, affecting physical characteristics like osmotic pressure and freezing point depression. Osmotic pressure arises from solvent molecules moving through a semipermeable membrane toward a solution with higher solute concentration, while freezing point depression occurs as solute particles disrupt the formation of the solid phase, lowering the solvent's freezing temperature. These properties enable calculations of molar masses and solution concentrations, fundamental in physical chemistry and biochemical applications.
Understanding Osmotic Pressure
Osmotic pressure is the pressure required to stop the flow of solvent molecules through a semipermeable membrane from a dilute to a concentrated solution, directly related to solute concentration. It can be quantified using the formula P = iMRT, where P is osmotic pressure, i is the van't Hoff factor, M is molarity, R is the gas constant, and T is temperature in Kelvin. Unlike freezing point depression, which depends on solute particle number affecting solvent solidification temperature, osmotic pressure specifically measures the force exerted by solutes to equalize chemical potential across membranes.
Explaining Freezing Point Depression
Freezing point depression occurs when a solute is dissolved in a solvent, causing the solution's freezing point to be lower than that of the pure solvent. This phenomenon results from the solute molecules disrupting the solvent's ability to form a solid lattice, thereby requiring a lower temperature to freeze. The extent of freezing point depression is directly proportional to the molal concentration of the solute particles, making it a colligative property used in determining molar masses and characterizing solutions.
Key Differences: Osmotic Pressure vs Freezing Point Depression
Osmotic pressure measures the force required to prevent solvent flow through a semipermeable membrane due to solute concentration differences, while freezing point depression is the lowering of a solvent's freezing temperature caused by solute addition. Osmotic pressure depends on solute concentration and temperature, directly affecting solvent movement, whereas freezing point depression quantifies colligative properties and is independent of solute type but relies on solute particle number. Both phenomena illustrate solvent-solute interactions but differ fundamentally in physical manifestation and measurement principles.
Molecular Basis Behind Both Phenomena
Osmotic pressure and freezing point depression both arise from colligative properties of solutions, depending on solute particle concentration rather than their identity. Osmotic pressure results from solvent molecules moving across a semipermeable membrane to equalize solute concentration, driven by the chemical potential difference at the molecular level. Freezing point depression occurs as solute particles disrupt the orderly arrangement of solvent molecules necessary for solidification, lowering the temperature at which the solvent crystallizes.
Mathematical Formulations and Comparisons
Osmotic pressure (p) is mathematically expressed by the formula p = iMRT, where i is the van't Hoff factor, M is the molarity, R is the gas constant, and T is the temperature in Kelvin. Freezing point depression (DTf) is given by DTf = iKf m, with Kf representing the cryoscopic constant and m the molality of the solution. Both phenomena depend on the colligative properties of solutions and the van't Hoff factor but differ in their dependence on concentration units and physical effects--osmotic pressure relates to solvent flow through a membrane, while freezing point depression involves lowering the phase change temperature.
Factors Influencing Osmotic Pressure and Freezing Point Depression
Osmotic pressure depends primarily on solute concentration, temperature, and the nature of the solvent, with higher solute concentrations and temperatures increasing osmotic pressure. Freezing point depression is influenced by the number of dissolved particles (solute concentration), the solvent type, and the presence of electrolytes, which disrupt the solvent's crystallization process. Both properties are colligative, relying on solute particle quantity rather than identity, yet osmotic pressure is more sensitive to temperature changes compared to freezing point depression.
Real-World Applications and Examples
Osmotic pressure plays a crucial role in medical treatments such as intravenous fluid administration, ensuring proper cellular hydration and nutrient balance. Freezing point depression is widely applied in de-icing roads and preserving food by lowering the freezing temperature through salt or antifreeze additives. Both phenomena are essential in industrial processes like water purification and cryopreservation, where controlling solution properties impacts efficiency and safety.
Measurement Techniques for Both Properties
Osmotic pressure is typically measured using osmometry techniques such as membrane osmometry or vapor pressure osmometry, which quantify the pressure required to prevent solvent flow across a semipermeable membrane. Freezing point depression is commonly determined through cryoscopic methods, where the temperature at which a solvent freezes is measured before and after solute addition using differential scanning calorimetry (DSC) or traditional freezing point apparatus. Both methods rely on precise temperature control and concentration measurements to accurately analyze colligative properties of solutions.
Summary and Implications for Chemistry and Industry
Osmotic pressure and freezing point depression are colligative properties that depend on solute particle concentration rather than their identity, crucial in determining solution behavior in chemistry and industrial processes. Osmotic pressure impacts fields like pharmaceuticals and water purification by controlling solvent flow through membranes, while freezing point depression is vital for antifreeze formulations and food preservation by lowering solution freezing points. Understanding these properties allows chemists and engineers to manipulate solution properties for optimized reactions, product stability, and process efficiency across various applications.
Osmotic pressure Infographic
 libterm.com
libterm.com