Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium with its liquid or solid phase at a given temperature. It provides critical insight into the volatility and evaporation rate of a substance, influencing processes such as distillation, refrigeration, and weather patterns. Explore the rest of the article to understand how vapor pressure impacts your everyday environment and industrial applications.
Table of Comparison
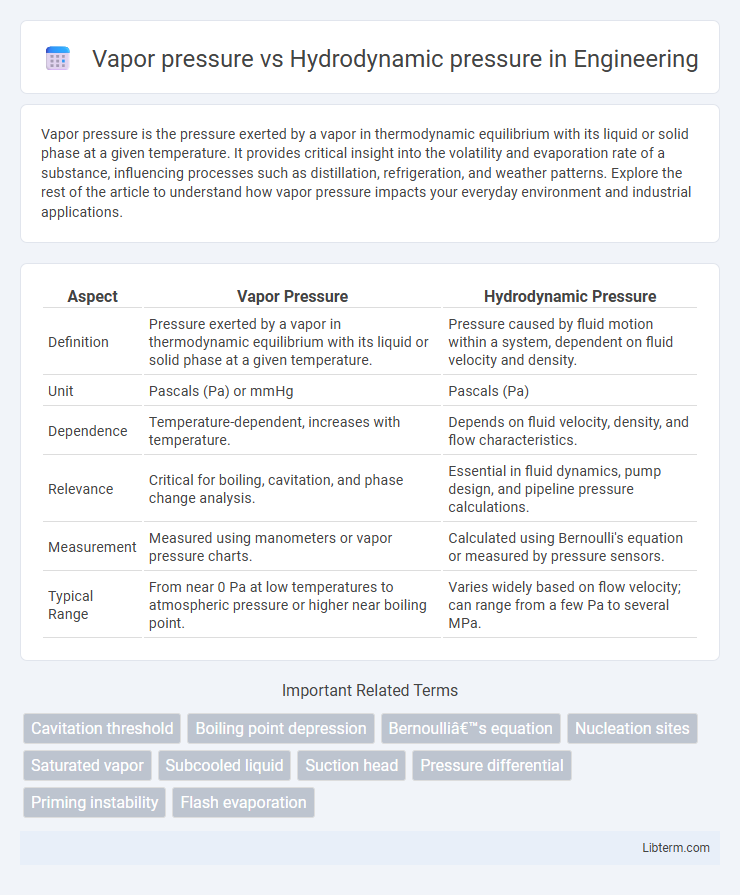
| Aspect | Vapor Pressure | Hydrodynamic Pressure |
|---|---|---|
| Definition | Pressure exerted by a vapor in thermodynamic equilibrium with its liquid or solid phase at a given temperature. | Pressure caused by fluid motion within a system, dependent on fluid velocity and density. |
| Unit | Pascals (Pa) or mmHg | Pascals (Pa) |
| Dependence | Temperature-dependent, increases with temperature. | Depends on fluid velocity, density, and flow characteristics. |
| Relevance | Critical for boiling, cavitation, and phase change analysis. | Essential in fluid dynamics, pump design, and pipeline pressure calculations. |
| Measurement | Measured using manometers or vapor pressure charts. | Calculated using Bernoulli's equation or measured by pressure sensors. |
| Typical Range | From near 0 Pa at low temperatures to atmospheric pressure or higher near boiling point. | Varies widely based on flow velocity; can range from a few Pa to several MPa. |
Understanding Vapor Pressure
Vapor pressure is the pressure exerted by a vapor in equilibrium with its liquid at a given temperature and is critical for predicting phase changes such as boiling and evaporation. It differs fundamentally from hydrodynamic pressure, which is the pressure exerted by a fluid in motion due to its velocity and density. Understanding vapor pressure enables accurate design in processes like distillation, refrigeration, and chemical engineering where controlling vapor-liquid equilibria is essential.
Defining Hydrodynamic Pressure
Hydrodynamic pressure refers to the pressure exerted by a fluid in motion, influenced by velocity and fluid density according to Bernoulli's principle. It plays a critical role in fluid dynamics, affecting flow behavior in pipes, channels, and around submerged surfaces. Unlike vapor pressure, which is the pressure at which a liquid vaporizes at a given temperature, hydrodynamic pressure depends on the fluid's kinetic energy and external forces acting on the fluid system.
Physical Principles of Vapor and Hydrodynamic Pressures
Vapor pressure represents the equilibrium pressure exerted by a vapor in contact with its liquid phase at a given temperature, driven by molecular evaporation and condensation rates. Hydrodynamic pressure arises from fluid motion and is governed by the Navier-Stokes equations, reflecting the mechanical force exerted by a fluid in motion or at rest within a system. Understanding the distinct origins--thermodynamic phase equilibrium for vapor pressure versus fluid dynamics and momentum transfer for hydrodynamic pressure--is essential in applications like cavitation, boiling, and fluid flow analysis.
Measurement Techniques: Vapor vs Hydrodynamic Pressure
Vapor pressure measurement techniques include isoteniscope, static method, and dynamic method using pressure sensors or manometers to determine the equilibrium vapor pressure at a specific temperature. Hydrodynamic pressure is typically measured using pitot tubes, pressure transducers, or manometers in fluid flow systems to capture the pressure exerted by moving fluids. Accurate vapor pressure readings require controlling temperature and avoiding condensation, while hydrodynamic pressure measurements emphasize velocity profiles and fluid density for precise flow characterization.
Factors Influencing Vapor Pressure
Vapor pressure depends primarily on temperature, the nature of the liquid, and atmospheric pressure, with higher temperatures increasing molecular evaporation rates and thus vapor pressure. Purity and intermolecular forces also impact vapor pressure since stronger cohesive forces in a liquid reduce the tendency of molecules to escape into the vapor phase. In contrast, hydrodynamic pressure relates to the physical pressure exerted by a fluid in motion, influenced by fluid velocity, density, and flow conditions rather than temperature or molecular properties.
Factors Affecting Hydrodynamic Pressure
Hydrodynamic pressure depends primarily on fluid velocity, density, and flow geometry, with increases in flow speed or fluid density raising the pressure exerted by the moving fluid. Pipe diameter, flow rate, and fluid viscosity also significantly influence hydrodynamic pressure by altering resistance and turbulence within the system. Unlike vapor pressure, which is temperature-dependent and related to phase change, hydrodynamic pressure varies with the dynamic conditions of fluid motion in pipelines or open channels.
Key Differences Between Vapor and Hydrodynamic Pressure
Vapor pressure is the pressure exerted by a vapor in equilibrium with its liquid or solid phase at a given temperature, while hydrodynamic pressure refers to the pressure within a fluid in motion due to the fluid's velocity and height. Vapor pressure depends primarily on temperature and the nature of the liquid, indicating the tendency of molecules to escape into the vapor phase, whereas hydrodynamic pressure is influenced by fluid velocity, density, and gravitational forces, described by Bernoulli's equation. Key differences include that vapor pressure is a thermodynamic property related to phase change and vaporization, whereas hydrodynamic pressure is a mechanical force acting within flowing fluids.
Applications in Engineering and Science
Vapor pressure is crucial in designing pressure vessels and boilers, as it determines the temperature at which liquids vaporize, impacting phase changes and safety protocols. Hydrodynamic pressure plays a vital role in fluid mechanics applications, influencing pump design, pipe flow, and aerodynamic assessments by quantifying pressure exerted by fluid motion. Both pressures are essential in chemical engineering processes, where vapor pressure affects distillation and evaporation, while hydrodynamic pressure governs fluid transport and flow stability.
Implications for Fluid Systems Design
Vapor pressure directly influences the risk of cavitation in fluid systems by determining the threshold at which liquid phases vaporize, causing pressure drops and potential damage to pumps and pipelines. Hydrodynamic pressure, governed by fluid velocity and system geometry, controls the overall flow dynamics and structural integrity within these systems. Understanding the interplay between vapor pressure and hydrodynamic pressure is critical for optimizing pump selection, preventing flow blockages, and ensuring reliable operation under varying temperature and pressure conditions.
Safety Considerations: Vapor Pressure and Hydrodynamic Contexts
Vapor pressure is critical in safety assessments as it determines the likelihood of a liquid to vaporize and form potentially explosive mixtures, especially in confined spaces. Hydrodynamic pressure relates to the fluid's motion and impact forces, influencing the structural integrity of pipelines and containment systems under dynamic conditions. Understanding both pressures is essential for designing fail-safe mechanisms that prevent leaks, explosions, and equipment failure in chemical processing and fluid transport industries.
Vapor pressure Infographic
 libterm.com
libterm.com