Galvanic corrosion occurs when two different metals are electrically connected in a corrosive environment, causing one metal to deteriorate faster than it would alone. This electrochemical process depends on factors such as metal type, electrolyte presence, and surface area differences, making material selection critical in design. Explore the rest of the article to understand how to prevent galvanic corrosion and protect your metal structures effectively.
Table of Comparison
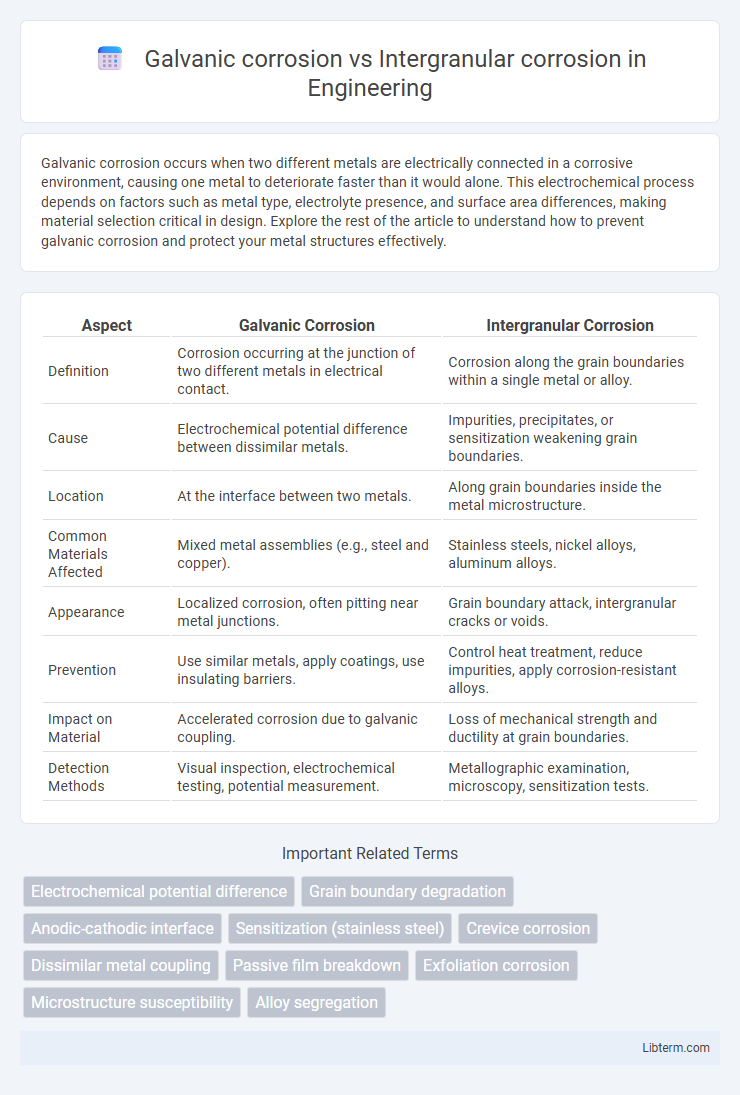
| Aspect | Galvanic Corrosion | Intergranular Corrosion |
|---|---|---|
| Definition | Corrosion occurring at the junction of two different metals in electrical contact. | Corrosion along the grain boundaries within a single metal or alloy. |
| Cause | Electrochemical potential difference between dissimilar metals. | Impurities, precipitates, or sensitization weakening grain boundaries. |
| Location | At the interface between two metals. | Along grain boundaries inside the metal microstructure. |
| Common Materials Affected | Mixed metal assemblies (e.g., steel and copper). | Stainless steels, nickel alloys, aluminum alloys. |
| Appearance | Localized corrosion, often pitting near metal junctions. | Grain boundary attack, intergranular cracks or voids. |
| Prevention | Use similar metals, apply coatings, use insulating barriers. | Control heat treatment, reduce impurities, apply corrosion-resistant alloys. |
| Impact on Material | Accelerated corrosion due to galvanic coupling. | Loss of mechanical strength and ductility at grain boundaries. |
| Detection Methods | Visual inspection, electrochemical testing, potential measurement. | Metallographic examination, microscopy, sensitization tests. |
Introduction to Galvanic and Intergranular Corrosion
Galvanic corrosion occurs when two dissimilar metals are electrically connected in the presence of an electrolyte, causing the more anodic metal to corrode preferentially. Intergranular corrosion targets the grain boundaries of a metal, often triggered by chemical or microstructural changes during processes like welding or heat treatment. Both types of corrosion significantly affect material integrity but involve different mechanisms and environments.
Fundamental Principles of Corrosion
Galvanic corrosion occurs when two dissimilar metals with different electrochemical potentials are electrically connected in a corrosive electrolyte, causing the more anodic metal to corrode preferentially. Intergranular corrosion arises from chemical or microstructural heterogeneities along grain boundaries, leading to localized attack and weakening of the metal. Both types of corrosion involve electrochemical processes but differ fundamentally in their initiation mechanisms and affected microstructural regions.
What is Galvanic Corrosion?
Galvanic corrosion occurs when two dissimilar metals are electrically connected in the presence of an electrolyte, causing the more anodic metal to corrode faster than it would alone. This electrochemical process leads to accelerated material degradation, often observed in metal joints, pipelines, and marine environments. Unlike intergranular corrosion, which targets grain boundaries within a single metal, galvanic corrosion depends on the interaction between different metals.
What is Intergranular Corrosion?
Intergranular corrosion is a localized attack occurring along the grain boundaries of a metal, often triggered by the precipitation of harmful phases such as chromium carbides in stainless steels. This type of corrosion compromises the structural integrity without significant surface deterioration, making it particularly insidious in aerospace and nuclear applications. Unlike galvanic corrosion, which involves electrochemical interaction between dissimilar metals, intergranular corrosion is driven by metallurgical factors within a single material.
Causes and Mechanisms of Galvanic Corrosion
Galvanic corrosion occurs when two dissimilar metals with different electrochemical potentials are electrically connected in an electrolyte, causing the more anodic metal to corrode preferentially. The mechanism involves electron flow from the anodic metal to the cathodic metal through the metallic connection, while ionic conduction occurs through the electrolyte, accelerating metal dissolution at the anode. Intergranular corrosion, in contrast, happens along grain boundaries due to localized chemical or structural heterogeneities, but galvanic corrosion specifically depends on the electrochemical potential difference and conductive electrolyte presence.
Causes and Mechanisms of Intergranular Corrosion
Intergranular corrosion occurs when grain boundaries in a metal become chemically or electrochemically different from the grain interiors, often due to impurity segregation or precipitation of secondary phases such as carbides. This localized attack primarily arises from sensitization in stainless steels, where chromium carbide formation at grain boundaries depletes chromium, reducing corrosion resistance in those regions. In contrast, galvanic corrosion results from electrochemical potential differences between two dissimilar metals or phases, causing accelerated corrosion at the anodic site.
Material Susceptibility: Galvanic vs Intergranular Corrosion
Galvanic corrosion occurs when two dissimilar metals with different electrode potentials are in electrical contact within a corrosive electrolyte, causing the less noble metal to corrode preferentially. Intergranular corrosion primarily affects susceptible alloys like stainless steels or aluminum, where grain boundaries become anodic sites due to localized depletion of alloying elements such as chromium or other precipitates. Material susceptibility to galvanic corrosion depends on the electrochemical potential difference between metals, whereas intergranular corrosion susceptibility hinges on microstructural factors such as grain boundary chemistry and heat treatment history.
Real-world Examples and Case Studies
Galvanic corrosion commonly occurs in marine environments where steel bolts are coupled with aluminum components, leading to rapid deterioration of the anodic aluminum due to the electrochemical potential difference, as seen in ship hulls and offshore platforms. Intergranular corrosion is frequently observed in stainless steel welded structures, such as chemical processing plants, where chromium carbide precipitates along grain boundaries cause localized attack and compromise material integrity. Case studies highlight a naval ship hull failure attributed to galvanic corrosion between copper piping and steel hull plates, while petrochemical refinery incidents reveal intergranular corrosion in sensitized stainless steel heat exchangers causing leaks and operational downtime.
Prevention and Mitigation Strategies
Galvanic corrosion prevention involves using insulating materials such as coatings or gaskets to electrically separate dissimilar metals and applying cathodic protection methods like sacrificial anodes. Intergranular corrosion mitigation requires careful material selection, including low-carbon stainless steels or stabilized alloys, and proper heat treatment to avoid chromium carbide precipitation at grain boundaries. Regular maintenance and monitoring, such as corrosion inspections and environmental control, help reduce the risk and extent of both galvanic and intergranular corrosion.
Conclusion: Choosing Optimal Protection Against Corrosion
Selecting optimal protection against galvanic corrosion requires isolating dissimilar metals through coatings or dielectric barriers to prevent electrochemical reactions. For intergranular corrosion, employing corrosion-resistant alloys and proper heat treatment to stabilize grain boundaries effectively inhibits degradation. Tailoring protection strategies to the specific corrosion mechanism ensures enhanced longevity and structural integrity of materials in diverse environments.
Galvanic corrosion Infographic
 libterm.com
libterm.com