Eutrophication occurs when water bodies become enriched with excess nutrients, leading to dense algal blooms and oxygen depletion that threaten aquatic life. This process disrupts ecosystems, reduces biodiversity, and impacts water quality, creating challenges for environmental management and human health. Explore the rest of this article to understand how eutrophication affects your local waterways and what steps can be taken to mitigate its effects.
Table of Comparison
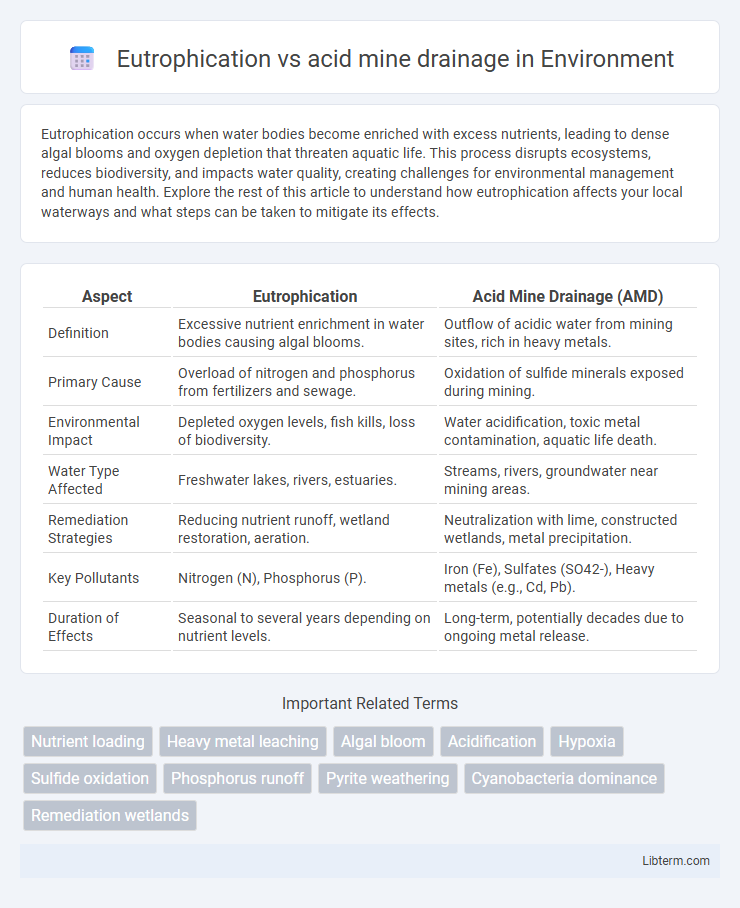
| Aspect | Eutrophication | Acid Mine Drainage (AMD) |
|---|---|---|
| Definition | Excessive nutrient enrichment in water bodies causing algal blooms. | Outflow of acidic water from mining sites, rich in heavy metals. |
| Primary Cause | Overload of nitrogen and phosphorus from fertilizers and sewage. | Oxidation of sulfide minerals exposed during mining. |
| Environmental Impact | Depleted oxygen levels, fish kills, loss of biodiversity. | Water acidification, toxic metal contamination, aquatic life death. |
| Water Type Affected | Freshwater lakes, rivers, estuaries. | Streams, rivers, groundwater near mining areas. |
| Remediation Strategies | Reducing nutrient runoff, wetland restoration, aeration. | Neutralization with lime, constructed wetlands, metal precipitation. |
| Key Pollutants | Nitrogen (N), Phosphorus (P). | Iron (Fe), Sulfates (SO42-), Heavy metals (e.g., Cd, Pb). |
| Duration of Effects | Seasonal to several years depending on nutrient levels. | Long-term, potentially decades due to ongoing metal release. |
Introduction to Eutrophication and Acid Mine Drainage
Eutrophication is the process by which water bodies become enriched with nutrients, primarily nitrogen and phosphorus, leading to excessive growth of algae and depletion of oxygen. Acid mine drainage occurs when sulfide minerals exposed during mining react with air and water, producing sulfuric acid that contaminates nearby water sources. Both phenomena cause severe environmental damage but differ in origin, with eutrophication largely driven by agricultural runoff and acid mine drainage linked to mining activities.
Defining Eutrophication: Causes and Processes
Eutrophication is the nutrient enrichment of aquatic ecosystems primarily caused by excessive nitrogen and phosphorus from agricultural runoff, wastewater discharge, and industrial effluents. This nutrient overload stimulates algal blooms, leading to oxygen depletion and harmful effects on aquatic life through hypoxia or anoxia. The primary processes involve nutrient loading, algal proliferation, organic matter decomposition, and subsequent oxygen consumption disrupting aquatic habitats.
Acid Mine Drainage: Origins and Chemical Mechanisms
Acid mine drainage (AMD) originates from the exposure of sulfide minerals, such as pyrite, to oxygen and water during mining activities, triggering intense oxidation reactions. This process produces sulfuric acid and releases heavy metals like iron, copper, and lead into surrounding waters, causing severe environmental contamination. The acidic conditions and metal toxicity from AMD profoundly disrupt aquatic ecosystems and pose challenges for water treatment and remediation efforts.
Key Differences Between Eutrophication and Acid Mine Drainage
Eutrophication primarily results from nutrient pollution, especially excess nitrogen and phosphorus, leading to oxygen depletion in aquatic ecosystems. Acid mine drainage occurs when sulfide minerals exposed by mining react with water and oxygen to produce sulfuric acid, drastically lowering pH and harming water quality. While eutrophication impacts biological productivity and oxygen levels, acid mine drainage mainly causes chemical acidity and metal contamination in water bodies.
Impact of Eutrophication on Aquatic Ecosystems
Eutrophication causes excessive nutrient enrichment in aquatic ecosystems, leading to algal blooms that reduce oxygen levels and create hypoxic conditions detrimental to fish and aquatic organisms. This nutrient overload disrupts food webs, decreases biodiversity, and results in the loss of habitat quality. The decline in water quality and increased mortality rates significantly impair ecosystem services such as fisheries and recreation.
Environmental Consequences of Acid Mine Drainage
Acid mine drainage (AMD) releases toxic heavy metals and sulfuric acid into water bodies, severely degrading aquatic ecosystems and contaminating drinking water sources. The low pH and high metal concentrations in AMD lead to bioaccumulation, destruction of fish populations, and loss of biodiversity. This environmental hazard contrasts with eutrophication, where nutrient overload primarily causes algal blooms and oxygen depletion rather than toxic metal pollution.
Human Activities Accelerating Eutrophication and Acid Mine Drainage
Human activities such as agricultural runoff rich in nitrogen and phosphorus fertilizers accelerate eutrophication by promoting excessive algal blooms that deplete oxygen in water bodies. Acid mine drainage occurs when mining exposes sulfide minerals to air and water, producing sulfuric acid that contaminates nearby ecosystems and water sources. Both processes threaten aquatic life and water quality, with eutrophication primarily driven by nutrient pollution and acid mine drainage linked to mining operations.
Monitoring and Identifying Affected Water Bodies
Monitoring eutrophication involves measuring nutrient concentrations, such as nitrogen and phosphorus, alongside indicators like chlorophyll-a and dissolved oxygen levels to identify algal blooms in affected water bodies. Acid mine drainage is detected by assessing parameters including low pH, elevated heavy metals (iron, copper, lead), and sulfate concentrations in downstream waters. Remote sensing technologies combined with in-situ water quality sensors enhance early identification and continuous monitoring of ecosystems impacted by both eutrophication and acid mine drainage.
Remediation Strategies: Prevention and Treatment Approaches
Eutrophication remediation focuses on reducing nutrient inputs through strategies like phosphorus and nitrogen load reduction, implementing buffer strips, and promoting wetland restoration to enhance natural filtration. Acid mine drainage treatment involves neutralizing acidic water using alkaline materials such as lime, as well as active and passive treatment systems including constructed wetlands and anoxic limestone drains to precipitate heavy metals. Both challenges require integrated watershed management and continuous monitoring to ensure long-term ecological recovery and water quality improvement.
Comparative Analysis: Long-term Effects and Management Solutions
Eutrophication causes nutrient overload in aquatic systems, leading to oxygen depletion and biodiversity loss, while acid mine drainage releases toxic metals and acidic water, severely harming ecosystems and water quality. Long-term effects of eutrophication include persistent algal blooms and dead zones, whereas acid mine drainage results in prolonged soil and water contamination requiring extensive remediation. Management solutions for eutrophication focus on nutrient input reduction and wetland restoration, contrasting with acid mine drainage strategies involving active neutralization, mine sealing, and advanced water treatment technologies.
Eutrophication Infographic
 libterm.com
libterm.com