Biodegradation is the natural process through which microorganisms break down organic materials into simpler substances, reducing environmental pollution. This eco-friendly mechanism plays a crucial role in waste management and soil health, making it essential in sustainable practices. Discover how biodegradation impacts your environment and contributes to a greener future by reading the full article.
Table of Comparison
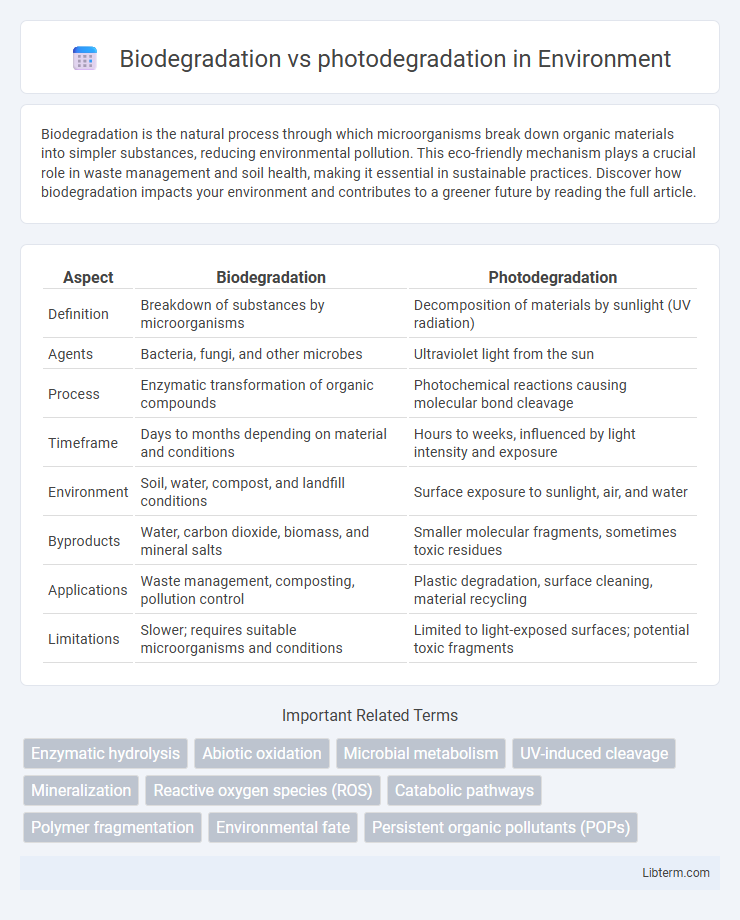
| Aspect | Biodegradation | Photodegradation |
|---|---|---|
| Definition | Breakdown of substances by microorganisms | Decomposition of materials by sunlight (UV radiation) |
| Agents | Bacteria, fungi, and other microbes | Ultraviolet light from the sun |
| Process | Enzymatic transformation of organic compounds | Photochemical reactions causing molecular bond cleavage |
| Timeframe | Days to months depending on material and conditions | Hours to weeks, influenced by light intensity and exposure |
| Environment | Soil, water, compost, and landfill conditions | Surface exposure to sunlight, air, and water |
| Byproducts | Water, carbon dioxide, biomass, and mineral salts | Smaller molecular fragments, sometimes toxic residues |
| Applications | Waste management, composting, pollution control | Plastic degradation, surface cleaning, material recycling |
| Limitations | Slower; requires suitable microorganisms and conditions | Limited to light-exposed surfaces; potential toxic fragments |
Understanding Biodegradation: Definition and Mechanisms
Biodegradation is the natural process by which microorganisms such as bacteria, fungi, and algae break down organic substances into simpler compounds, often resulting in water, carbon dioxide, and biomass. This mechanism relies on enzymatic reactions that metabolize complex polymers like plastics, pesticides, and hydrocarbons under aerobic or anaerobic conditions. Understanding the microbial pathways and environmental factors influencing biodegradation is essential for optimizing waste management and environmental remediation strategies.
Photodegradation Explained: Key Processes and Principles
Photodegradation involves the breakdown of materials through exposure to ultraviolet (UV) light, primarily from the sun, which triggers photochemical reactions that alter the molecular structure of polymers and organic compounds. Key processes include the absorption of UV rays by chromophores, leading to bond cleavage, free radical formation, and subsequent chain reactions that result in the material's fragmentation. Photodegradation plays a crucial role in environmental fate by accelerating the decomposition of plastics and pollutants, influencing ecosystems and waste management strategies.
Major Differences Between Biodegradation and Photodegradation
Biodegradation involves the breakdown of organic materials by microorganisms such as bacteria and fungi, resulting in the conversion of substances into natural elements like water, carbon dioxide, and biomass. Photodegradation relies on exposure to sunlight, particularly UV radiation, to chemically break down compounds through photochemical reactions, often leading to fragmentation and loss of molecular integrity. The major differences between biodegradation and photodegradation lie in the biological versus abiotic mechanisms, the involvement of living organisms in biodegradation, and the requirement of sunlight as an energy source in photodegradation.
Environmental Impact of Biodegradation
Biodegradation involves the breakdown of organic materials by microorganisms, resulting in less harmful byproducts that integrate back into natural ecosystems, reducing pollution and waste accumulation. This process minimizes toxic residue and supports soil fertility, promoting sustainable waste management and ecosystem health. In contrast to photodegradation, biodegradation significantly lowers the environmental footprint by preventing the release of microplastics and chemical pollutants.
Environmental Impact of Photodegradation
Photodegradation breaks down pollutants using sunlight-driven chemical reactions, potentially producing reactive intermediates that can harm aquatic ecosystems. Unlike biodegradation, which involves microbial activity converting substances into benign end products like carbon dioxide and water, photodegradation may generate partial degradation products that accumulate and persist in the environment. The environmental impact of photodegradation depends on the nature of the photoproducts formed, with some contributing to toxicity and others facilitating further degradation under natural sunlight exposure.
Factors Influencing the Rate of Biodegradation
The rate of biodegradation is influenced by factors such as the presence of specific microorganisms, temperature, pH levels, oxygen availability, and the chemical structure of the material being decomposed. Materials with simpler molecular structures and higher biodegradability tend to decompose faster, especially in environments with optimal moisture and nutrient conditions. In contrast, photodegradation relies more on light exposure and wavelength intensity, making biodegradation more dependent on biological and environmental parameters.
Factors Affecting Photodegradation Efficiency
Photodegradation efficiency depends on factors such as the intensity and wavelength of light, presence of photosensitizers, and environmental conditions like temperature and humidity. The chemical structure of the pollutant, including its susceptibility to light-induced reactions, significantly influences degradation rates. Surface area and exposure duration to light sources also play crucial roles in determining the overall photodegradation efficiency.
Real-world Applications: Biodegradation in Waste Management
Biodegradation plays a critical role in waste management by using microorganisms to break down organic waste into non-toxic components, reducing landfill volume and pollutant release. This natural process supports composting facilities and bioreactors, enhancing the recycling of biodegradable materials like food scraps and agricultural residues. Photodegradation, in contrast, primarily aids in breaking down synthetic pollutants through sunlight exposure, with limited direct application in organic waste treatment systems.
Innovations in Photodegradation Technologies
Innovations in photodegradation technologies harness advanced photocatalysts such as titanium dioxide nanoparticles and graphene-based composites to accelerate the breakdown of pollutants under visible light, significantly enhancing efficiency compared to traditional biodegradation methods. These cutting-edge materials enable rapid degradation of complex organic compounds, including dyes and pharmaceuticals, by generating reactive oxygen species that cleave chemical bonds without harmful residues. Emerging developments in solar-powered photodegradation reactors and novel semiconductor heterojunctions further optimize light absorption and electron-hole separation, offering sustainable and scalable solutions for environmental remediation.
Choosing Between Biodegradation and Photodegradation: Considerations and Future Trends
Choosing between biodegradation and photodegradation depends on factors such as environmental conditions, material composition, and desired degradation timeframe. Biodegradation leverages microbial activity ideal for organic waste in soil or aqueous environments, while photodegradation relies on UV light exposure, making it suitable for surface applications and degrading pollutants in air or on surfaces. Emerging trends emphasize combining both processes with advanced catalysts and engineered microbes to enhance degradation efficiency and sustainability across various ecosystems.
Biodegradation Infographic
 libterm.com
libterm.com