Bioequivalence ensures that two pharmaceutical products release their active ingredients into the bloodstream at similar rates and extents, confirming comparable bioavailability. Therapeutic equivalence indicates that products not only are bioequivalent but also produce the same clinical effect and safety profile when administered under the same conditions. Discover how understanding these concepts can impact Your treatment options as you read the rest of this article.
Table of Comparison
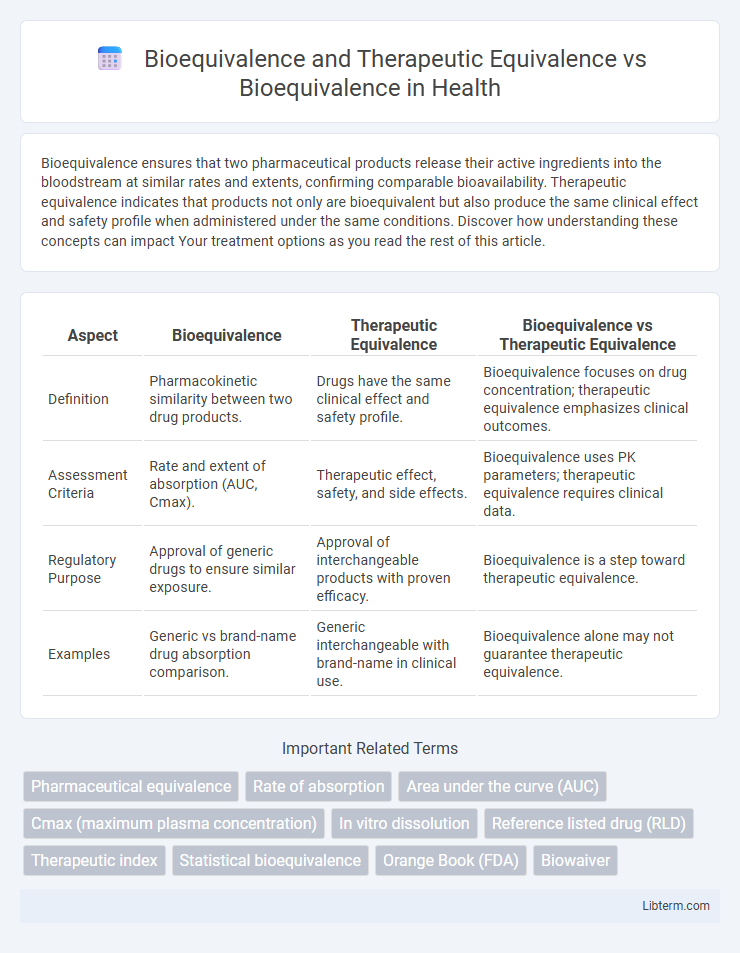
| Aspect | Bioequivalence | Therapeutic Equivalence | Bioequivalence vs Therapeutic Equivalence |
|---|---|---|---|
| Definition | Pharmacokinetic similarity between two drug products. | Drugs have the same clinical effect and safety profile. | Bioequivalence focuses on drug concentration; therapeutic equivalence emphasizes clinical outcomes. |
| Assessment Criteria | Rate and extent of absorption (AUC, Cmax). | Therapeutic effect, safety, and side effects. | Bioequivalence uses PK parameters; therapeutic equivalence requires clinical data. |
| Regulatory Purpose | Approval of generic drugs to ensure similar exposure. | Approval of interchangeable products with proven efficacy. | Bioequivalence is a step toward therapeutic equivalence. |
| Examples | Generic vs brand-name drug absorption comparison. | Generic interchangeable with brand-name in clinical use. | Bioequivalence alone may not guarantee therapeutic equivalence. |
Introduction to Bioequivalence and Therapeutic Equivalence
Bioequivalence refers to the comparison of the bioavailability of the same active pharmaceutical ingredient in two drug products to ensure equivalent absorption and therapeutic effect. Therapeutic equivalence encompasses bioequivalence but also requires pharmaceutical equivalence and similar safety and efficacy profiles, confirming that a generic drug performs in the same manner as the branded counterpart. Understanding these concepts is essential for regulatory approval and ensuring patient safety when substituting generic medications.
Defining Bioequivalence: Core Concepts
Bioequivalence refers to the absence of a significant difference in the bioavailability of two pharmaceutical products when administered at the same molar dose under similar conditions, ensuring comparable rate and extent of absorption. Therapeutic equivalence encompasses bioequivalence but also requires pharmaceutical equivalence and acceptable labeling, indicating the products are expected to have the same clinical effect and safety profile. Defining bioequivalence focuses on pharmacokinetic measures such as C_max (maximum concentration) and AUC (area under the curve) to establish that the generic and reference drugs deliver the same active ingredient into the bloodstream in a similar timeframe.
Therapeutic Equivalence: A Broader Perspective
Therapeutic equivalence encompasses bioequivalence but extends further to ensure that pharmaceutical products have the same clinical effect and safety profile when administered under the conditions specified in the labeling. While bioequivalence focuses on comparable bioavailability parameters such as Cmax and AUC, therapeutic equivalence evaluates clinical outcomes, ensuring interchangeability in therapeutic use. Regulatory agencies, including the FDA, require both bioequivalence and pharmaceutical equivalence before designating a generic drug as therapeutically equivalent to a brand-name counterpart.
Bioequivalence vs Therapeutic Equivalence: Key Differences
Bioequivalence refers to the absence of significant difference in the rate and extent of absorption of the active pharmaceutical ingredient between two drug products, ensuring similar bioavailability. Therapeutic equivalence implies that two products are bioequivalent and also pharmaceutically equivalent, meaning they contain the same active ingredient, dosage form, strength, and meet safety and efficacy standards, producing the same clinical effect. The key difference lies in therapeutic equivalence encompassing both bioequivalence and pharmaceutical equivalence, whereas bioequivalence solely addresses the pharmacokinetic parameters of drug absorption.
Regulatory Guidelines for Bioequivalence Assessment
Regulatory guidelines for bioequivalence assessment, such as those from the FDA and EMA, emphasize the comparison of pharmacokinetic parameters like Cmax and AUC to demonstrate equivalent systemic drug exposure between generic and reference products. Therapeutic equivalence requires both bioequivalence and pharmaceutical equivalence, ensuring comparable clinical efficacy and safety. Bioequivalence focuses on the similarity in absorption and bioavailability, while therapeutic equivalence includes additional criteria such as dosage form and labeling compliance.
Criteria for Establishing Therapeutic Equivalence
Therapeutic equivalence requires that a generic drug not only demonstrate bioequivalence, showing comparable bioavailability to the reference drug, but also meet criteria for pharmaceutical equivalence and safety, efficacy, and labeling consistency. The FDA establishes therapeutic equivalence by evaluating bioequivalence studies alongside assessments of dosage form, strength, and quality to ensure substitutable clinical effects. Regulatory standards for therapeutic equivalence include rigorous in vivo pharmacokinetic parameters such as AUC and Cmax within 80-125% confidence intervals, ensuring interchangeable therapeutic outcomes.
The Role of Bioequivalence in Generic Drug Approval
Bioequivalence plays a crucial role in generic drug approval by demonstrating that the generic product releases the same amount of active ingredient into the bloodstream at the same rate as the brand-name counterpart, ensuring similar efficacy and safety profiles. Regulatory agencies like the FDA require bioequivalence studies to confirm that pharmacokinetic parameters such as Cmax (maximum concentration) and AUC (area under the curve) fall within an acceptable range, typically 80-125%. Therapeutic equivalence builds on bioequivalence by also confirming that the generic drug is pharmaceutically equivalent and interchangeable with the reference product, solidifying its approval for clinical use.
Clinical Significance of Therapeutic Equivalence
Therapeutic equivalence ensures that pharmaceutical products not only have the same bioavailability as their reference products but also deliver identical clinical effects and safety profiles, which is critical for assured patient outcomes. While bioequivalence focuses on comparable rate and extent of absorption between generic and brand-name drugs, therapeutic equivalence emphasizes the drug's clinical efficacy and safety in real-world use. The clinical significance of therapeutic equivalence lies in confirming that interchangeable medications produce the same therapeutic results, minimizing risks of adverse reactions or therapeutic failures.
Challenges in Demonstrating Bioequivalence and Therapeutic Equivalence
Challenges in demonstrating bioequivalence include variability in drug absorption, complex pharmacokinetics, and ensuring consistent systemic exposure between generic and reference products. Therapeutic equivalence adds complexity by requiring evidence of comparable clinical efficacy and safety, not just pharmacokinetic similarity, which is difficult when dealing with drugs with narrow therapeutic indices or variable patient responses. Regulatory standards demand rigorous in vivo and in vitro testing, making it challenging to establish both bioequivalence and therapeutic equivalence simultaneously for complex formulations.
Future Perspectives in Drug Equivalence Evaluation
Emerging technologies such as artificial intelligence and advanced pharmacokinetic modeling are transforming bioequivalence and therapeutic equivalence assessments by enabling more precise prediction of drug performance in diverse populations. Regulatory frameworks are evolving towards incorporating real-world evidence and in vitro-in vivo correlation (IVIVC) to enhance the robustness of equivalence evaluations. Future drug equivalence studies will likely prioritize personalized medicine approaches and adaptive trial designs to optimize therapeutic outcomes and streamline generic drug approvals.
Bioequivalence and Therapeutic Equivalence Infographic
 libterm.com
libterm.com