Calorimetry measures the heat transfer associated with chemical reactions or physical changes, providing critical insights into energy changes and thermodynamic properties. Precise calorimetric techniques enable you to determine specific heat capacities, enthalpy changes, and reaction kinetics essential for scientific research and industrial applications. Explore the rest of this article to understand the principles, types, and practical uses of calorimetry.
Table of Comparison
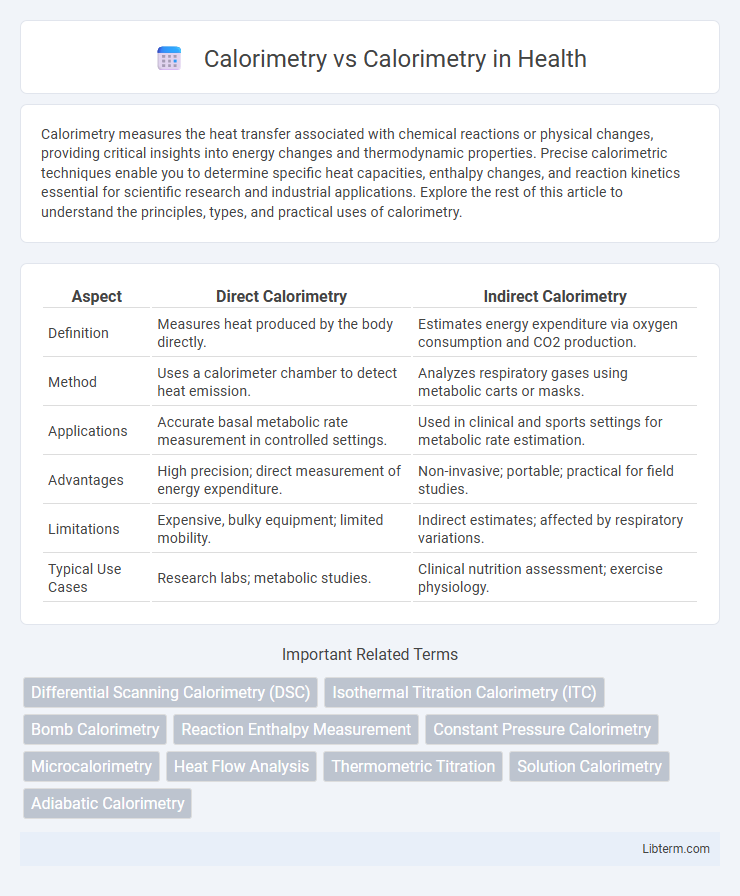
| Aspect | Direct Calorimetry | Indirect Calorimetry |
|---|---|---|
| Definition | Measures heat produced by the body directly. | Estimates energy expenditure via oxygen consumption and CO2 production. |
| Method | Uses a calorimeter chamber to detect heat emission. | Analyzes respiratory gases using metabolic carts or masks. |
| Applications | Accurate basal metabolic rate measurement in controlled settings. | Used in clinical and sports settings for metabolic rate estimation. |
| Advantages | High precision; direct measurement of energy expenditure. | Non-invasive; portable; practical for field studies. |
| Limitations | Expensive, bulky equipment; limited mobility. | Indirect estimates; affected by respiratory variations. |
| Typical Use Cases | Research labs; metabolic studies. | Clinical nutrition assessment; exercise physiology. |
Introduction to Calorimetry
Calorimetry is the science of measuring the heat exchanged during chemical reactions or physical changes to determine energy transfer. It involves using devices called calorimeters, which provide precise temperature change data to calculate enthalpy changes. Understanding the principles of calorimetry is essential for fields such as thermodynamics, biochemistry, and material science to analyze energy flow and reaction dynamics.
Understanding the Basics of Calorimetry
Calorimetry measures the heat transfer during physical or chemical processes, quantifying energy changes in substances. Key concepts include specific heat capacity, heat flow, and calorimeter types such as bomb and coffee cup calorimeters. Understanding these fundamentals enables accurate determination of enthalpy changes and thermodynamic properties.
Types of Calorimetry Explained
Types of calorimetry include constant-pressure calorimetry, constant-volume calorimetry, and differential scanning calorimetry, each measuring heat transfer under specific conditions. Constant-pressure calorimetry, often performed with a coffee cup calorimeter, tracks enthalpy changes during chemical reactions occurring at atmospheric pressure. Differential scanning calorimetry analyzes phase transitions and heat capacity by measuring temperature differences between a sample and reference under controlled heating rates.
Principles Behind Calorimetric Measurements
Calorimetry relies on the principle of heat transfer to measure the amount of thermal energy released or absorbed during physical or chemical processes, enabling precise determinations of enthalpy changes. The technique involves detecting temperature variations in a controlled environment, typically using a calorimeter, where the heat exchange between the system and surroundings is quantified. Accurate calorimetric measurements depend on calibrating the device, understanding the specific heat capacities involved, and minimizing heat loss to ensure reliable energy data.
Key Differences in Calorimetry Methods
Constant-pressure calorimetry measures heat changes at atmospheric pressure using a coffee cup calorimeter, ideal for reactions in solution, whereas constant-volume calorimetry employs a bomb calorimeter to gauge heat at fixed volume, typically for combustion processes. Differential scanning calorimetry (DSC) records heat flow changes as a function of temperature, enabling analysis of phase transitions and material properties. Isothermal titration calorimetry (ITC) quantifies heat during molecular interactions at constant temperature, providing precise binding affinity and thermodynamic data.
Applications of Calorimetry in Science
Calorimetry plays a crucial role in thermodynamics by measuring the heat exchange during chemical reactions, phase transitions, and physical changes in materials. Applications of calorimetry in science include studying enzyme kinetics in biochemistry, determining the heat capacity of substances in physics, and analyzing combustion reactions for energy content assessment in environmental science. Advanced techniques such as differential scanning calorimetry (DSC) and isothermal titration calorimetry (ITC) provide precise data essential for drug development, material science, and metabolic studies.
Advantages and Limitations of Calorimetry Techniques
Calorimetry techniques offer precise measurements of heat flow and thermal properties, enabling detailed analysis of material phase transitions, reaction kinetics, and thermodynamic parameters. Key advantages include high sensitivity to small thermal changes and the ability to analyze both physical and chemical processes under controlled conditions. Limitations involve sample size constraints, potential interference from environmental factors, and the requirement for calibration standards to ensure accurate quantitative data.
Comparative Analysis: Calorimetry Methods
Comparative analysis of calorimetry methods reveals differential precision and application scopes, with differential scanning calorimetry (DSC) offering high sensitivity for phase transitions and heat capacity measurements, while isothermal titration calorimetry (ITC) excels in quantifying molecular interaction energetics such as binding constants and enthalpy changes. Bomb calorimetry is preferred for assessing heat of combustion in materials, delivering robust results under constant volume conditions, contrasting with the constant pressure environment of solution calorimetry suited for reaction enthalpies. Selection depends on sample type, desired thermodynamic parameters, and operational constraints, emphasizing tailored method choice for optimal data fidelity in thermal analysis.
Recent Advances in Calorimetry Technologies
Recent advances in calorimetry technologies emphasize enhanced sensitivity and real-time analysis through microcalorimetry and nanocalorimetry, enabling precise thermal measurements at microscopic scales. Innovations in differential scanning calorimetry (DSC) have improved resolution and speed, facilitating detailed phase transition studies in complex materials. Integration of calorimetry with digital data processing allows for automated, high-throughput thermal characterization, advancing applications in pharmaceuticals, materials science, and biochemical research.
Conclusion: Choosing the Right Calorimetry Approach
Selecting the appropriate calorimetry method depends on the specific experimental goals, sample characteristics, and desired sensitivity. Differential scanning calorimetry (DSC) is ideal for analyzing phase transitions and heat capacity with high precision, while isothermal titration calorimetry (ITC) excels in studying molecular interactions by directly measuring binding thermodynamics. Understanding the strengths and limitations of each calorimetry technique ensures accurate thermal analysis and optimal data interpretation for biochemical and material science applications.
Calorimetry Infographic
 libterm.com
libterm.com