Carboxyhemoglobin forms when carbon monoxide binds to hemoglobin in your blood, reducing oxygen transport and leading to tissue hypoxia. This compound's presence is a key indicator of carbon monoxide poisoning, which can cause serious health effects or even death if untreated. Learn more about how carboxyhemoglobin impacts your body and how to recognize exposure symptoms in the rest of this article.
Table of Comparison
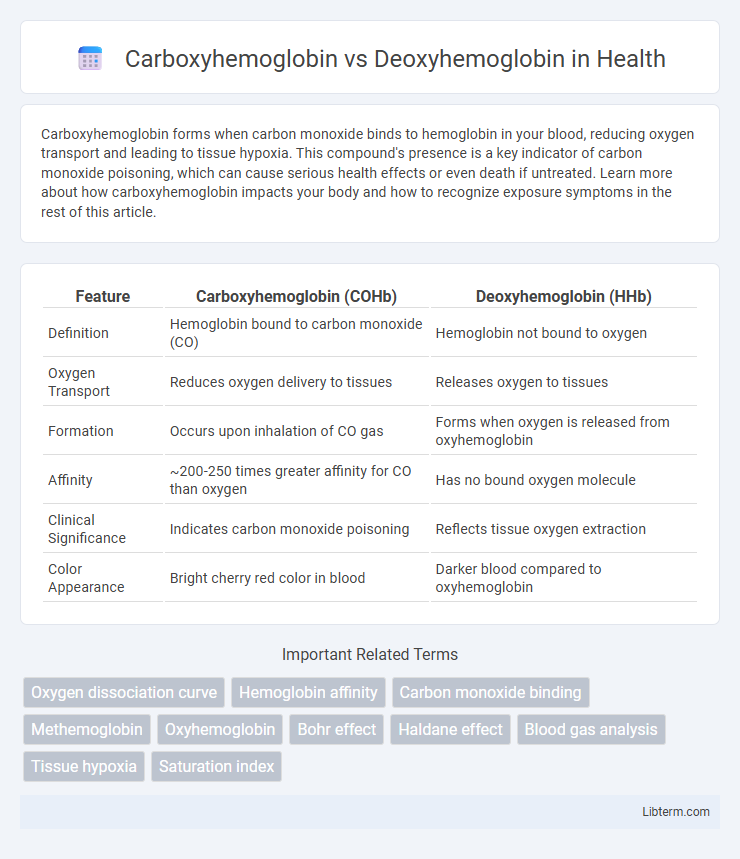
| Feature | Carboxyhemoglobin (COHb) | Deoxyhemoglobin (HHb) |
|---|---|---|
| Definition | Hemoglobin bound to carbon monoxide (CO) | Hemoglobin not bound to oxygen |
| Oxygen Transport | Reduces oxygen delivery to tissues | Releases oxygen to tissues |
| Formation | Occurs upon inhalation of CO gas | Forms when oxygen is released from oxyhemoglobin |
| Affinity | ~200-250 times greater affinity for CO than oxygen | Has no bound oxygen molecule |
| Clinical Significance | Indicates carbon monoxide poisoning | Reflects tissue oxygen extraction |
| Color Appearance | Bright cherry red color in blood | Darker blood compared to oxyhemoglobin |
Introduction to Hemoglobin Variants
Carboxyhemoglobin forms when hemoglobin binds with carbon monoxide, significantly reducing oxygen transport capacity due to its high affinity, while deoxyhemoglobin represents hemoglobin without oxygen, critical for oxygen delivery in tissues. These hemoglobin variants differ in molecular binding states, influencing their roles in respiratory physiology and clinical diagnostics. Understanding the distinction between carboxyhemoglobin and deoxyhemoglobin is essential for interpreting hemoglobin function under various physiological and pathological conditions.
Structure of Carboxyhemoglobin
Carboxyhemoglobin forms when carbon monoxide (CO) binds with hemoglobin's heme iron, producing a stable complex that alters oxygen transport. The carbon monoxide molecule binds with a much higher affinity than oxygen, causing a conformational change that differs from deoxyhemoglobin, where iron is in the ferrous state but not bound to oxygen. This structural modification reduces oxygen release to tissues, leading to tissue hypoxia and toxicity.
Structure of Deoxyhemoglobin
Deoxyhemoglobin is a tetrameric protein structure composed of two alpha and two beta globin chains, each containing a heme group without bound oxygen, resulting in a tense (T) state conformation. This T state facilitates oxygen release by stabilizing the deoxygenated form and promoting cooperative oxygen binding when oxygen is available. In contrast, carboxyhemoglobin forms when carbon monoxide binds to the heme iron with high affinity, preventing oxygen binding and significantly altering hemoglobin's oxygen transport function.
Formation Mechanisms
Carboxyhemoglobin forms when carbon monoxide (CO) binds tightly to the iron in hemoglobin, outcompeting oxygen due to its 200-250 times higher affinity, thereby preventing oxygen transport. Deoxyhemoglobin results from the release of oxygen molecules from oxyhemoglobin, typically occurring in peripheral tissues where oxygen partial pressure is low. The formation of carboxyhemoglobin involves a direct binding mechanism at the heme site, while deoxyhemoglobin formation involves oxygen dissociation driven by tissue oxygen demand and pH changes.
Oxygen Binding Affinity Differences
Carboxyhemoglobin exhibits a significantly higher oxygen binding affinity compared to deoxyhemoglobin, due to carbon monoxide's strong affinity for the heme iron, which is approximately 200-250 times greater than oxygen's affinity. This binding reduces oxygen transport efficiency by preventing oxygen release to tissues, leading to hypoxia. Deoxyhemoglobin, in contrast, has lower oxygen affinity, allowing for easier oxygen unloading essential for cellular respiration.
Physiological Roles in the Body
Carboxyhemoglobin forms when carbon monoxide binds to hemoglobin with a much higher affinity than oxygen, preventing oxygen delivery to tissues and impairing cellular respiration. Deoxyhemoglobin, in contrast, represents hemoglobin lacking bound oxygen, playing a crucial role in releasing oxygen from red blood cells to meet metabolic demands in peripheral tissues. The physiological balance between these forms is essential for maintaining efficient oxygen transport and preventing hypoxic damage caused by carbon monoxide exposure.
Clinical Implications and Toxicity
Carboxyhemoglobin forms when carbon monoxide binds with hemoglobin, reducing oxygen delivery and causing tissue hypoxia, posing significant risks in CO poisoning cases. Deoxyhemoglobin represents hemoglobin not bound to oxygen, reflecting the venous blood oxygen state and its levels are crucial for assessing oxygen dissociation and delivery efficiency. Elevated carboxyhemoglobin levels require prompt clinical intervention to prevent severe toxicity and organ damage, while monitoring deoxyhemoglobin aids in evaluating respiratory and circulatory function in critical care.
Detection and Measurement Methods
Carboxyhemoglobin is typically detected and measured using co-oximetry, which differentiates it from oxyhemoglobin and deoxyhemoglobin based on distinct light absorption spectra. Deoxyhemoglobin measurement frequently employs pulse oximetry or spectrophotometric methods, utilizing its unique absorption characteristics in the visible and near-infrared range. Advanced techniques like gas chromatography-mass spectrometry (GC-MS) and blood gas analyzers provide precise quantification of carboxyhemoglobin and deoxyhemoglobin levels in clinical diagnostics.
Health Effects and Symptoms
Carboxyhemoglobin forms when carbon monoxide binds with hemoglobin, significantly reducing oxygen delivery to tissues, leading to symptoms such as headache, dizziness, confusion, and in severe cases, loss of consciousness or death. Deoxyhemoglobin indicates hemoglobin without bound oxygen, commonly seen in tissues where oxygen is released, but elevated levels in the blood may signal hypoxemia, causing cyanosis, shortness of breath, and fatigue. The health impact of carboxyhemoglobin is acute toxicity requiring immediate medical intervention, while deoxyhemoglobin levels reflect oxygen deprivation typically managed by addressing underlying respiratory or circulatory conditions.
Prevention and Treatment Strategies
Preventing carboxyhemoglobin formation centers on reducing carbon monoxide (CO) exposure by ensuring proper ventilation and using CO detectors in homes and workplaces. Treatment strategies for elevated carboxyhemoglobin levels prioritize administering 100% oxygen or hyperbaric oxygen therapy to accelerate CO dissociation from hemoglobin and restore adequate oxygen delivery. In contrast, managing conditions involving deoxyhemoglobin primarily involves maintaining adequate oxygenation through supplemental oxygen and addressing underlying causes like respiratory or circulatory impairments.
Carboxyhemoglobin Infographic
 libterm.com
libterm.com