Schwann cells are essential glial cells in the peripheral nervous system responsible for the formation of the myelin sheath around nerve fibers, which facilitates rapid signal transmission. These cells also play a crucial role in nerve repair and regeneration after injury by guiding axonal growth. Discover how Schwann cells impact your nervous system function and explore their vital roles in this detailed article.
Table of Comparison
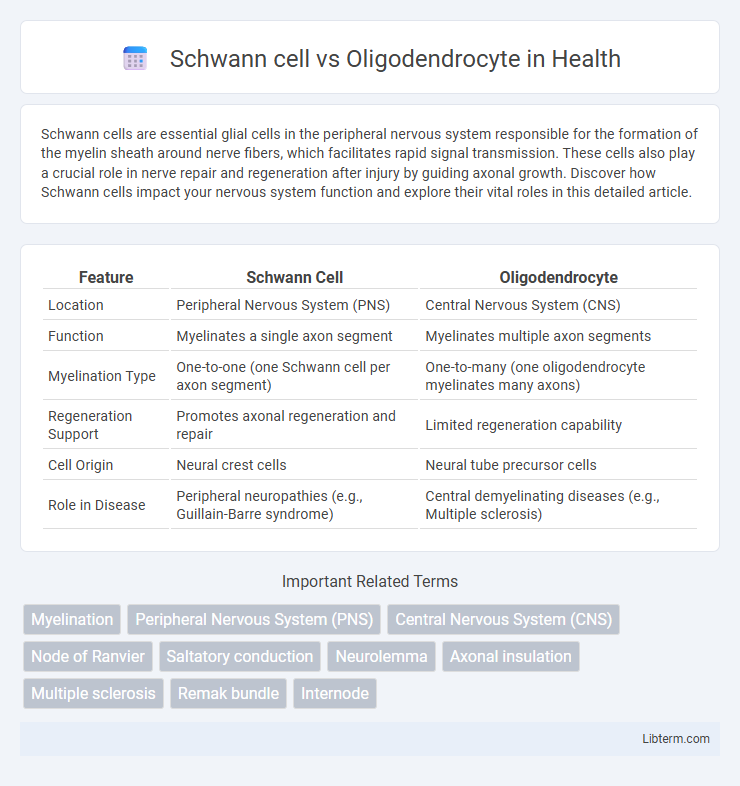
| Feature | Schwann Cell | Oligodendrocyte |
|---|---|---|
| Location | Peripheral Nervous System (PNS) | Central Nervous System (CNS) |
| Function | Myelinates a single axon segment | Myelinates multiple axon segments |
| Myelination Type | One-to-one (one Schwann cell per axon segment) | One-to-many (one oligodendrocyte myelinates many axons) |
| Regeneration Support | Promotes axonal regeneration and repair | Limited regeneration capability |
| Cell Origin | Neural crest cells | Neural tube precursor cells |
| Role in Disease | Peripheral neuropathies (e.g., Guillain-Barre syndrome) | Central demyelinating diseases (e.g., Multiple sclerosis) |
Introduction to Glial Cells in the Nervous System
Schwann cells and oligodendrocytes are key glial cells responsible for myelination in the peripheral and central nervous systems, respectively. Schwann cells envelop a single axon segment in the peripheral nervous system, promoting rapid electrical conduction, while oligodendrocytes extend multiple processes to myelinate several axons simultaneously in the central nervous system. Both cell types play crucial roles in neural support, insulation, and repair, essential for maintaining effective neuronal communication.
Overview: Schwann Cells and Oligodendrocytes
Schwann cells and oligodendrocytes are glial cells responsible for myelinating axons in the peripheral and central nervous systems, respectively. Schwann cells wrap individual axons in the peripheral nervous system, providing insulation and facilitating rapid nerve impulse transmission, while oligodendrocytes extend their processes to myelinate multiple axons simultaneously in the central nervous system. Both cell types are essential for maintaining neural function and supporting axonal integrity but differ in their location, structure, and capacity to regenerate nerve fibers.
Origin and Development of Schwann Cells vs Oligodendrocytes
Schwann cells originate from the neural crest during embryonic development and are responsible for myelinating peripheral nervous system (PNS) axons, while oligodendrocytes derive from oligodendrocyte precursor cells in the neuroectoderm of the central nervous system (CNS). Schwann cell development involves migration along axons followed by differentiation into myelinating or non-myelinating phenotypes, regulated by transcription factors like Sox10 and Krox20. Oligodendrocyte maturation is driven by signaling pathways such as PDGF and IGF-1, promoting the proliferation and differentiation of oligodendrocyte precursor cells into myelinating oligodendrocytes within the CNS.
Structural Differences and Morphology
Schwann cells and oligodendrocytes both produce myelin in the nervous system but differ structurally and morphologically. Schwann cells are elongated, wrapping a single axon segment in the peripheral nervous system, forming a distinct myelin sheath by encasing one neuron. Oligodendrocytes, found in the central nervous system, have multiple processes extending from the cell body, enabling them to myelinate several axon segments simultaneously.
Myelination in the Peripheral vs Central Nervous System
Schwann cells are responsible for myelination in the peripheral nervous system (PNS), forming sheath segments around single axons to enhance nerve signal conduction. In contrast, oligodendrocytes myelinate multiple axons simultaneously within the central nervous system (CNS), providing faster and more efficient electrical insulation. The distinct cellular mechanisms and geographic localization of Schwann cells and oligodendrocytes reflect their specialized roles in nerve regeneration and functional recovery.
Functional Roles and Mechanisms
Schwann cells, located in the peripheral nervous system, facilitate rapid nerve impulse conduction by forming myelin sheaths around a single axon segment through their enwrapping and wrapping mechanisms. Oligodendrocytes, found in the central nervous system, extend multiple processes to myelinate several axons simultaneously, enhancing signal transmission speed and providing metabolic support. Both cell types contribute to nerve regeneration, but Schwann cells actively promote axonal repair, whereas oligodendrocytes play a more limited role in regeneration and predominantly support axonal stability and function.
Key Molecular Markers and Genetic Regulation
Schwann cells express key molecular markers including myelin protein zero (MPZ), peripheral myelin protein 22 (PMP22), and S100 calcium-binding protein, which are critical for peripheral nerve myelination, while oligodendrocytes predominantly express myelin basic protein (MBP), proteolipid protein 1 (PLP1), and Olig2 transcription factor, essential for central nervous system myelination. The genetic regulation of Schwann cells involves the transcription factor Sox10, which governs differentiation and myelin gene expression, whereas oligodendrocyte development relies on Olig1/2, Nkx2.2, and Sox10 in a tightly regulated network driving oligodendrocyte precursor cell maturation and myelin sheath formation. Differences in gene regulatory networks highlight the distinct molecular mechanisms underlying peripheral versus central myelin production.
Involvement in Nervous System Diseases
Schwann cells and oligodendrocytes both play critical roles in the myelination of axons in the peripheral and central nervous systems, respectively, with dysfunctions linked to distinct neurological diseases. Schwann cell abnormalities contribute to peripheral neuropathies such as Charcot-Marie-Tooth disease and Guillain-Barre syndrome by impairing nerve regeneration and altering myelin sheath integrity. Oligodendrocyte damage is central to multiple sclerosis and leukodystrophies, where demyelination leads to disrupted nerve signaling and progressive neurodegeneration.
Regeneration and Repair Capabilities
Schwann cells enable robust peripheral nerve regeneration by forming myelin sheaths around axons and promoting axonal growth through secretion of neurotrophic factors and guidance of regrowing fibers. Oligodendrocytes, primarily responsible for central nervous system myelination, exhibit limited regenerative capacity and do not support axonal repair effectively after injury. Their restricted ability to remyelinate and the inhibitory environment of the central nervous system contribute to poor repair outcomes compared to Schwann cells in peripheral nerves.
Future Research and Therapeutic Potential
Schwann cells and oligodendrocytes play crucial roles in myelination within the peripheral and central nervous systems, respectively, offering promising avenues for regenerative therapies in demyelinating diseases such as multiple sclerosis and peripheral neuropathies. Future research focuses on enhancing Schwann cell transplantation techniques and oligodendrocyte precursor cell (OPC) differentiation to promote remyelination and functional recovery. Advances in gene editing and biomaterials aim to optimize cell-based therapies, potentially revolutionizing treatment outcomes for neurodegenerative and traumatic injuries.
Schwann cell Infographic
 libterm.com
libterm.com