Carboxyhemoglobin forms when carbon monoxide binds with hemoglobin, reducing oxygen transport in the blood and causing hypoxia. This binding decreases the blood's capacity to deliver oxygen to tissues, leading to symptoms like headache, dizziness, and in severe cases, loss of consciousness. Discover more about the causes, symptoms, and treatments of carboxyhemoglobin exposure in the rest of this article.
Table of Comparison
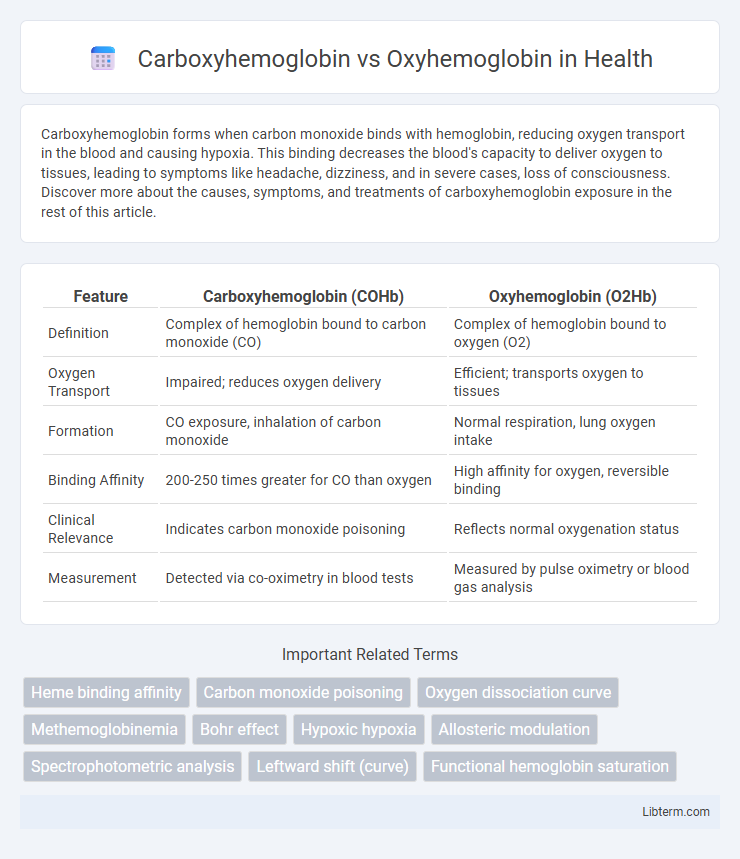
| Feature | Carboxyhemoglobin (COHb) | Oxyhemoglobin (O2Hb) |
|---|---|---|
| Definition | Complex of hemoglobin bound to carbon monoxide (CO) | Complex of hemoglobin bound to oxygen (O2) |
| Oxygen Transport | Impaired; reduces oxygen delivery | Efficient; transports oxygen to tissues |
| Formation | CO exposure, inhalation of carbon monoxide | Normal respiration, lung oxygen intake |
| Binding Affinity | 200-250 times greater for CO than oxygen | High affinity for oxygen, reversible binding |
| Clinical Relevance | Indicates carbon monoxide poisoning | Reflects normal oxygenation status |
| Measurement | Detected via co-oximetry in blood tests | Measured by pulse oximetry or blood gas analysis |
Introduction to Hemoglobin Complexes
Carboxyhemoglobin forms when carbon monoxide binds to the iron atom in hemoglobin with an affinity approximately 240 times greater than oxygen, significantly impairing oxygen transport. Oxyhemoglobin, the predominant form under normal physiological conditions, efficiently carries oxygen from the lungs to tissues by binding oxygen molecules reversibly to hemoglobin. The structural differences between these complexes affect their affinity for gases, with carboxyhemoglobin causing a leftward shift in the oxygen-hemoglobin dissociation curve, reducing oxygen delivery to vital organs.
What is Carboxyhemoglobin?
Carboxyhemoglobin is a complex formed when carbon monoxide (CO) binds with hemoglobin in red blood cells, significantly reducing oxygen transport capacity. Unlike oxyhemoglobin, which carries oxygen molecules to tissues, carboxyhemoglobin prevents oxygen binding, leading to cellular hypoxia and potential carbon monoxide poisoning. Its presence is measured to assess CO exposure and the severity of poisoning cases.
Understanding Oxyhemoglobin
Oxyhemoglobin is a complex formed when hemoglobin binds reversibly with oxygen molecules in the lungs, facilitating efficient oxygen transport to tissues throughout the body. This binding induces a conformational change in hemoglobin, increasing its affinity for oxygen and enabling oxygen delivery under varying partial pressures. Understanding oxyhemoglobin's role is crucial for comprehending respiratory physiology and the impact of factors like pH, temperature, and carbon dioxide on oxygen release at the cellular level.
Chemical Structure Differences
Carboxyhemoglobin forms when carbon monoxide binds to the iron ion in the heme group of hemoglobin, creating a stable complex that prevents oxygen binding, unlike oxyhemoglobin where oxygen binds reversibly to the iron ion. The chemical structure of carboxyhemoglobin involves a stronger Fe-CO bond, approximately 200 times more stable than the Fe-O2 bond in oxyhemoglobin, which inhibits oxygen transport. This difference in ligand binding affinity results in impaired oxygen delivery to tissues and altered hemoglobin conformation.
Mechanisms of Oxygen and Carbon Monoxide Binding
Carboxyhemoglobin forms when carbon monoxide (CO) binds to the iron atom in hemoglobin with approximately 240 times the affinity of oxygen, preventing oxygen from attaching to the same site. Oxyhemoglobin results from oxygen binding reversibly to the iron within the heme group, allowing efficient oxygen transport and release in tissues. The competitive binding of CO versus oxygen significantly impairs oxygen delivery due to carboxyhemoglobin's stability and slow dissociation rate, which disrupts normal hemoglobin function.
Physiological Functions and Roles
Carboxyhemoglobin forms when carbon monoxide binds with hemoglobin, impeding oxygen transport and reducing oxygen delivery to tissues, which disrupts cellular respiration and causes hypoxia. In contrast, oxyhemoglobin carries oxygen from the lungs to tissues, facilitating efficient cellular metabolism and energy production. The physiological role of oxyhemoglobin is critical for sustaining aerobic functions, whereas carboxyhemoglobin's presence is toxic and diminishes the blood's oxygen-carrying capacity.
Health Implications and Toxicity
Carboxyhemoglobin forms when carbon monoxide binds with hemoglobin, reducing oxygen transport and resulting in hypoxic tissue injury, posing significant health risks such as headaches, dizziness, and in severe cases, death. Oxyhemoglobin carries oxygen efficiently to tissues, supporting normal cellular respiration and metabolic functions without toxicity. Elevated carboxyhemoglobin levels disrupt oxygen delivery, leading to cellular hypoxia and potentially fatal carbon monoxide poisoning, necessitating immediate medical intervention.
Diagnostic Methods for Detection
Carboxyhemoglobin and oxyhemoglobin levels are primarily measured using co-oximetry, which differentiates these hemoglobin species based on their distinct light absorption spectra. Pulse CO-oximeters provide non-invasive, real-time monitoring of carboxyhemoglobin, essential for detecting carbon monoxide poisoning swiftly. Blood gas analyzers equipped with multi-wavelength spectrophotometry enhance diagnostic accuracy by quantifying both oxyhemoglobin saturation and carboxyhemoglobin percentage directly from arterial blood samples.
Treatment Strategies and Interventions
Treatment strategies for carboxyhemoglobin poisoning prioritize rapid administration of 100% oxygen and hyperbaric oxygen therapy to displace carbon monoxide from hemoglobin, restoring oxygen delivery. Oxyhemoglobin levels are supported by ensuring adequate oxygen supplementation and addressing underlying respiratory or circulatory issues through mechanical ventilation or medications. Early intervention targeting the reduction of carboxyhemoglobin concentration and enhancement of oxyhemoglobin formation is critical for minimizing tissue hypoxia and improving patient outcomes.
Summary: Key Differences and Clinical Significance
Carboxyhemoglobin forms when carbon monoxide binds to hemoglobin with a 200-250 times greater affinity than oxygen, preventing oxygen transport and causing tissue hypoxia, whereas oxyhemoglobin carries oxygen from the lungs to tissues efficiently. Clinically, elevated carboxyhemoglobin levels indicate carbon monoxide poisoning, a life-threatening condition requiring immediate treatment with high-flow oxygen or hyperbaric oxygen therapy. Understanding the differences is critical for diagnosis and management of hypoxic conditions and carbon monoxide toxicity.
Carboxyhemoglobin Infographic
 libterm.com
libterm.com