Deoxyhemoglobin is the form of hemoglobin without bound oxygen, playing a vital role in transporting carbon dioxide and regulating blood flow through vasodilation. Its concentration influences oxygen delivery and can be measured for insights into tissue oxygenation status. Explore the rest of the article to understand how deoxyhemoglobin impacts your health and diagnostic processes.
Table of Comparison
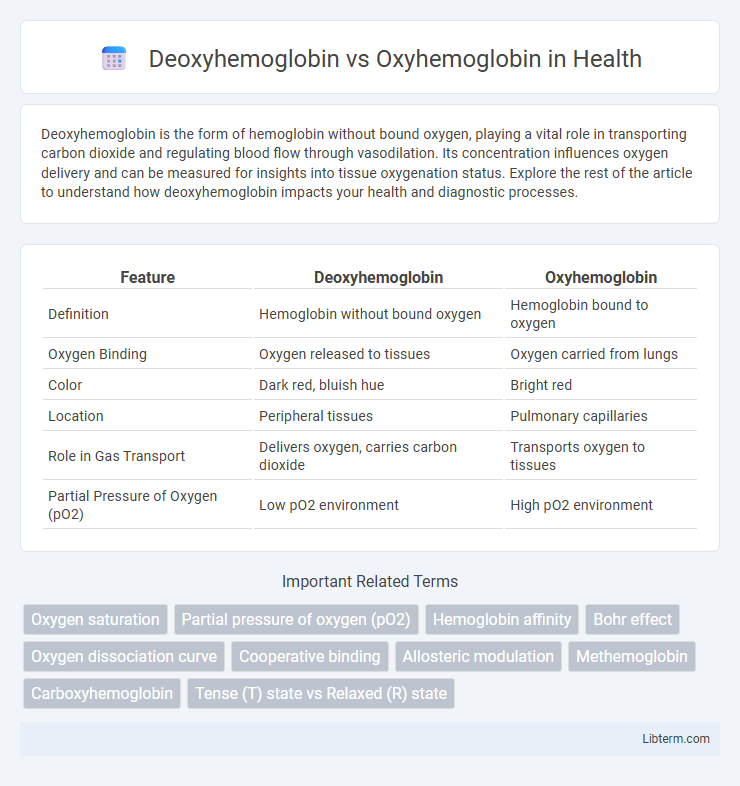
| Feature | Deoxyhemoglobin | Oxyhemoglobin |
|---|---|---|
| Definition | Hemoglobin without bound oxygen | Hemoglobin bound to oxygen |
| Oxygen Binding | Oxygen released to tissues | Oxygen carried from lungs |
| Color | Dark red, bluish hue | Bright red |
| Location | Peripheral tissues | Pulmonary capillaries |
| Role in Gas Transport | Delivers oxygen, carries carbon dioxide | Transports oxygen to tissues |
| Partial Pressure of Oxygen (pO2) | Low pO2 environment | High pO2 environment |
Introduction to Hemoglobin and Its Forms
Hemoglobin is a complex iron-containing protein in red blood cells responsible for oxygen transport throughout the body. It exists primarily in two forms: oxyhemoglobin, where oxygen molecules bind to the iron atoms in hemoglobin, and deoxyhemoglobin, which lacks bound oxygen and appears in venous blood. The reversible binding capacity allows hemoglobin to efficiently pick up oxygen in the lungs and release it in tissues, maintaining vital metabolic functions.
Structural Differences: Deoxyhemoglobin vs Oxyhemoglobin
Deoxyhemoglobin and oxyhemoglobin differ structurally in the conformation of the hemoglobin molecule; deoxyhemoglobin has a tense (T) state with iron atoms slightly out of the heme plane, reducing oxygen affinity, while oxyhemoglobin adopts a relaxed (R) state where iron atoms move into the heme plane upon oxygen binding, increasing affinity. This conformational shift alters the quaternary structure of hemoglobin, enabling cooperative oxygen binding. The transition between these two states is critical for efficient oxygen transport and release in tissues.
Oxygen Binding Mechanism
Deoxyhemoglobin binds oxygen through a reversible interaction at the iron atom within its heme group, transitioning from a tense (T) state to a relaxed (R) state upon oxygen binding. This conformational change increases hemoglobin's affinity for additional oxygen molecules, facilitating cooperative binding. Oxyhemoglobin, formed after oxygen binding, transports oxygen efficiently in the bloodstream and releases it under lower partial pressures in tissues.
Allosteric Effects and Hemoglobin Function
Deoxyhemoglobin exhibits a tense (T) state with reduced oxygen affinity, facilitating oxygen release through allosteric effects that increase hemoglobin's capacity to bind protons, CO2, and 2,3-Bisphosphoglycerate (2,3-BPG). Oxyhemoglobin transitions to a relaxed (R) state, enhancing oxygen affinity and promoting oxygen uptake in the lungs while decreasing affinity for allosteric effectors, thus optimizing oxygen transport efficiency. The cooperative binding mechanism of hemoglobin, driven by allosteric conformational changes between T and R states, is fundamental to its function in oxygen delivery and tissue respiration.
Role in Oxygen Transport and Delivery
Deoxyhemoglobin and oxyhemoglobin play crucial roles in oxygen transport and delivery within the bloodstream. Oxyhemoglobin, formed when hemoglobin binds to oxygen in the lungs, efficiently carries oxygen to tissues, while deoxyhemoglobin results when oxygen is released to cells, facilitating carbon dioxide pickup for removal. The dynamic conversion between these two forms enables effective oxygen delivery and cellular respiration throughout the body.
Hemoglobin Oxygen Dissociation Curve
Deoxyhemoglobin and oxyhemoglobin exhibit distinct affinities for oxygen, reflected in the hemoglobin oxygen dissociation curve, which plots oxygen saturation against partial pressure of oxygen (pO2). Oxyhemoglobin forms at higher pO2 levels in the lungs, showing a sigmoidal curve due to cooperative binding, whereas deoxyhemoglobin predominates at lower pO2 in tissues, facilitating oxygen release. The steep slope of the curve at physiological pO2 enables efficient oxygen unloading by deoxyhemoglobin and loading by oxyhemoglobin, critical for tissue oxygen delivery.
Physiological Significance and Adaptations
Deoxyhemoglobin and oxyhemoglobin play crucial roles in oxygen transport and delivery, with oxyhemoglobin carrying oxygen from the lungs to tissues and deoxyhemoglobin returning to the lungs for reoxygenation. The physiological significance lies in their ability to efficiently bind and release oxygen depending on the partial pressure of oxygen and pH, facilitating cellular respiration. Adaptations such as the Bohr effect and 2,3-BPG concentration modulate hemoglobin's oxygen affinity, optimizing oxygen delivery during varying metabolic demands and environmental conditions.
Clinical Relevance: Disorders and Diagnostics
Deoxyhemoglobin and oxyhemoglobin levels are critical indicators in diagnosing and monitoring conditions such as hypoxemia, anemia, and carbon monoxide poisoning. Pulse oximetry measures the ratio of oxyhemoglobin to total hemoglobin to assess oxygen saturation, providing essential data for respiratory and cardiovascular disorders. Abnormal levels of deoxyhemoglobin can signify impaired oxygen delivery, prompting further investigation through arterial blood gas analysis and co-oximetry for accurate clinical assessment.
Deoxyhemoglobin and Oxyhemoglobin in Medical Imaging
Deoxyhemoglobin and oxyhemoglobin are critical in medical imaging, especially in functional MRI (fMRI), where they provide contrast based on blood oxygenation levels. Deoxyhemoglobin, paramagnetic and more prevalent in deoxygenated blood, causes local magnetic field inhomogeneities that decrease the MR signal, enabling detection of brain activity through blood oxygen level-dependent (BOLD) contrast. Oxyhemoglobin, diamagnetic and found in oxygen-rich blood, produces a stronger MR signal, making the differential between these two forms essential for mapping neural activation and vascular health.
Key Differences Summarized
Deoxyhemoglobin is hemoglobin without bound oxygen, exhibiting a tense (T) state that facilitates oxygen release, whereas oxyhemoglobin contains oxygen and adopts a relaxed (R) state enhancing oxygen transport in the bloodstream. The structural shift between deoxyhemoglobin and oxyhemoglobin involves conformational changes in the heme group and globin chains, affecting their affinity for oxygen. Key physiological differences include deoxyhemoglobin's role in oxygen unloading in peripheral tissues and oxyhemoglobin's function in oxygen delivery from the lungs to body cells.
Deoxyhemoglobin Infographic
 libterm.com
libterm.com