Sulfhemoglobin is an abnormal form of hemoglobin in which sulfur binds to the hemoglobin molecule, impairing its oxygen-carrying capacity and often causing a greenish or cyanotic discoloration of the blood. This condition can result from exposure to certain drugs or chemicals and may lead to symptoms such as cyanosis and hypoxia. Discover how sulfhemoglobin affects your health and what treatments are available by reading the rest of the article.
Table of Comparison
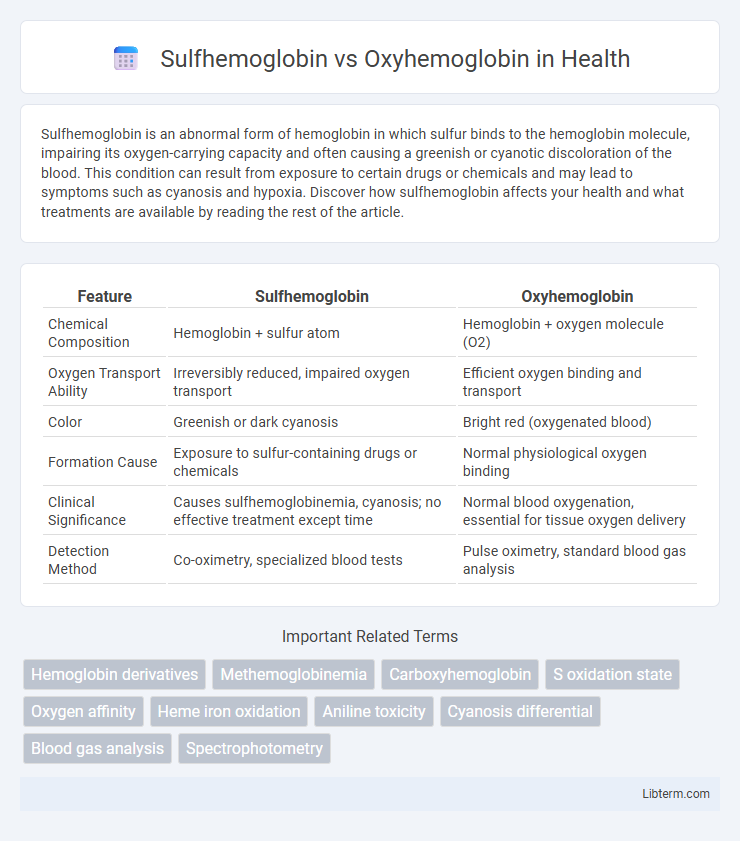
| Feature | Sulfhemoglobin | Oxyhemoglobin |
|---|---|---|
| Chemical Composition | Hemoglobin + sulfur atom | Hemoglobin + oxygen molecule (O2) |
| Oxygen Transport Ability | Irreversibly reduced, impaired oxygen transport | Efficient oxygen binding and transport |
| Color | Greenish or dark cyanosis | Bright red (oxygenated blood) |
| Formation Cause | Exposure to sulfur-containing drugs or chemicals | Normal physiological oxygen binding |
| Clinical Significance | Causes sulfhemoglobinemia, cyanosis; no effective treatment except time | Normal blood oxygenation, essential for tissue oxygen delivery |
| Detection Method | Co-oximetry, specialized blood tests | Pulse oximetry, standard blood gas analysis |
Introduction to Hemoglobin Variants
Sulfhemoglobin is a hemoglobin variant formed when sulfur atoms bind irreversibly to the hemoglobin molecule, impairing its oxygen-carrying capacity and resulting in cyanosis. Oxyhemoglobin represents the normal form of hemoglobin bound to oxygen, essential for efficient oxygen transport from the lungs to tissues. Understanding these variants is crucial for diagnosing and managing conditions related to impaired oxygen delivery and abnormal hemoglobin function.
What is Oxyhemoglobin?
Oxyhemoglobin is a complex formed when hemoglobin binds reversibly to oxygen molecules, facilitating efficient oxygen transport from the lungs to tissues. It exhibits a bright red color, indicating oxygen-rich blood, and plays a critical role in cellular respiration and metabolism. Unlike sulfhemoglobin, which contains sulfur and cannot bind oxygen effectively, oxyhemoglobin ensures optimal oxygen delivery and maintains physiological homeostasis.
What is Sulfhemoglobin?
Sulfhemoglobin is a rare, abnormal form of hemoglobin created when sulfur binds to the hemoglobin molecule, impairing its oxygen-carrying capacity. Unlike oxyhemoglobin, which efficiently transports oxygen to tissues, sulfhemoglobin cannot bind oxygen effectively, leading to hypoxia and cyanosis. This condition can result from exposure to certain drugs or chemicals, causing a distinct greenish pigment in the blood.
Structural Differences: Sulfhemoglobin vs Oxyhemoglobin
Sulfhemoglobin differs structurally from oxyhemoglobin by the irreversible incorporation of a sulfur atom into the heme moiety, creating a greenish pigment that cannot bind oxygen. Oxyhemoglobin contains iron in the ferrous (Fe2+) state bound reversibly to oxygen molecules, enabling efficient oxygen transport in the bloodstream. The presence of sulfur in sulfhemoglobin alters the heme's electronic configuration, reducing oxygen affinity and impairing hemoglobin function.
Oxygen Binding Capacity Comparison
Sulfhemoglobin significantly reduces oxygen binding capacity compared to oxyhemoglobin due to the irreversible incorporation of sulfur into the hemoglobin molecule, which inhibits its ability to carry oxygen efficiently. Oxyhemoglobin binds oxygen reversibly at the iron in heme groups, enabling effective oxygen transport, whereas sulfhemoglobin cannot bind oxygen, leading to impaired oxygen delivery to tissues. This reduction in oxygen binding capacity with sulfhemoglobinemia causes hypoxia despite normal or elevated oxygen partial pressures in the blood.
Causes and Formation of Sulfhemoglobin
Sulfhemoglobin forms when sulfur atoms irreversibly bind to the hemoglobin molecule, a process often triggered by exposure to certain drugs like sulfonamides, phenazopyridine, or hydrogen sulfide. Unlike oxyhemoglobin, which carries oxygen efficiently in the bloodstream, sulfhemoglobin cannot bind oxygen, leading to hypoxia and cyanosis. The formation of sulfhemoglobin results from oxidative damage and sulfur incorporation into the porphyrin ring of hemoglobin, causing a persistent alteration in hemoglobin's oxygen-carrying capacity.
Physiological Roles and Functions
Sulfhemoglobin forms when sulfur atoms irreversibly bind to hemoglobin, impairing oxygen delivery by reducing hemoglobin's affinity for oxygen and causing cyanosis without improving tissue oxygenation. Oxyhemoglobin is the oxygenated form of hemoglobin responsible for transporting oxygen from the lungs to peripheral tissues, facilitating aerobic metabolism and maintaining cellular respiration. The physiological role of oxyhemoglobin is crucial for sustaining life, while sulfhemoglobin represents a pathological state with limited oxygen-carrying capacity and no physiological benefit.
Detection and Diagnostic Methods
Sulfhemoglobin and oxyhemoglobin are distinguished through spectrophotometric analysis, where sulfhemoglobin shows an absorption peak around 620 nm, contrasting with oxyhemoglobin's peaks at 540 nm and 576 nm. Co-oximetry is the preferred diagnostic method for accurate quantification of sulfhemoglobin levels, as standard pulse oximetry cannot differentiate sulfhemoglobin from oxyhemoglobin. Confirmatory diagnosis often requires blood gas analysis combined with specialized hemoglobin electrophoresis or mass spectrometry to precisely identify sulfhemoglobin presence.
Clinical Significance and Health Implications
Sulfhemoglobin forms when sulfur binds irreversibly to hemoglobin, impairing its oxygen-carrying capacity and leading to cyanosis without hypoxemia, often seen in exposure to sulfur-containing drugs or toxins. Oxyhemoglobin represents normal hemoglobin bound to oxygen, essential for effective tissue oxygenation and cellular metabolism. Clinically, elevated sulfhemoglobin levels require prompt identification and discontinuation of causative agents to prevent chronic hypoxia, while oxyhemoglobin saturation guides critical assessments of respiratory and cardiovascular function.
Prevention and Management Strategies
Preventing sulfhemoglobinemia involves avoiding exposure to sulfur-containing drugs such as sulfonamides, phenazopyridine, and certain industrial chemicals, which can irreversibly bind to hemoglobin and impair oxygen transport. Management strategies for sulfhemoglobinemia include immediate discontinuation of the offending agent and supportive care, as there is no specific antidote and the condition often resolves with red blood cell turnover. In contrast, oxyhemoglobin monitoring focuses on maintaining adequate oxygenation through supplemental oxygen therapy and treating underlying respiratory or cardiac conditions to optimize hemoglobin's oxygen-carrying capacity.
Sulfhemoglobin Infographic
 libterm.com
libterm.com