A placebo is an inactive substance or treatment designed to mimic the appearance of a real drug without containing any therapeutic ingredients. It plays a crucial role in clinical trials by helping to measure the actual effectiveness of new medications through comparison. Discover how understanding placebos can impact your perspective on medical treatments by reading the rest of this article.
Table of Comparison
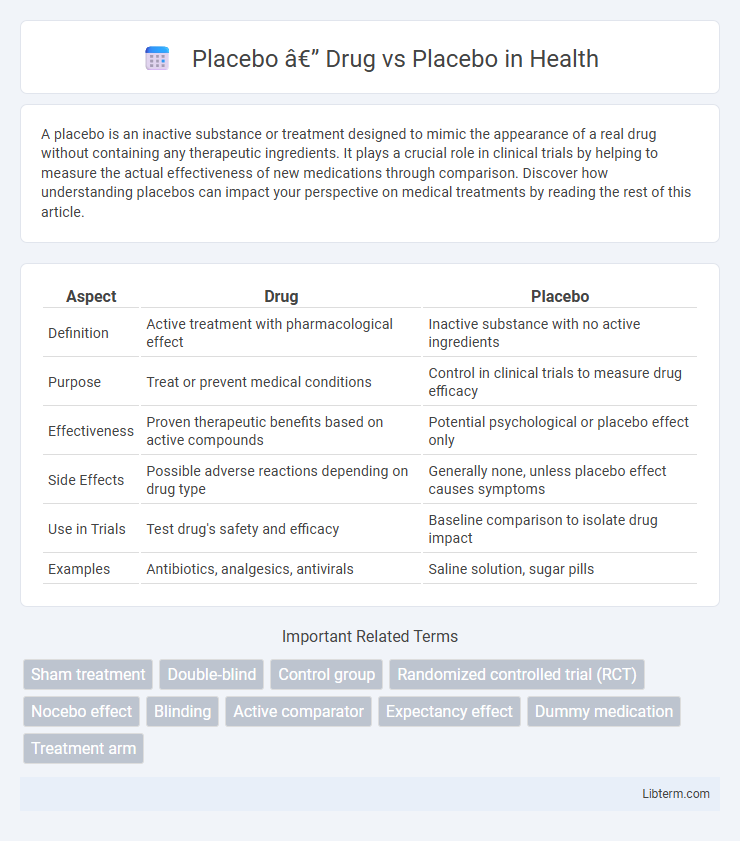
| Aspect | Drug | Placebo |
|---|---|---|
| Definition | Active treatment with pharmacological effect | Inactive substance with no active ingredients |
| Purpose | Treat or prevent medical conditions | Control in clinical trials to measure drug efficacy |
| Effectiveness | Proven therapeutic benefits based on active compounds | Potential psychological or placebo effect only |
| Side Effects | Possible adverse reactions depending on drug type | Generally none, unless placebo effect causes symptoms |
| Use in Trials | Test drug's safety and efficacy | Baseline comparison to isolate drug impact |
| Examples | Antibiotics, analgesics, antivirals | Saline solution, sugar pills |
Understanding the Placebo Effect
The placebo effect occurs when patients experience real improvements in symptoms after receiving a non-active treatment, such as a sugar pill, due to their expectations of healing. Clinical trials use drug vs placebo comparisons to isolate a medication's true efficacy by differentiating actual pharmacological benefits from psychological or psychosomatic responses. Understanding the placebo effect is critical for accurate drug evaluation, ensuring that improvements are attributed to the drug itself rather than patients' belief in treatment.
Defining Placebo in Clinical Trials
A placebo is an inert substance or treatment used in clinical trials to evaluate the efficacy of a new drug by serving as a control. It mimics the experimental drug's appearance but lacks active pharmacological ingredients, helping isolate the drug's true therapeutic effects from psychological or physiological responses. Placebos are crucial for identifying the placebo effect, ensuring objective assessment of drug safety and effectiveness.
The Science Behind Placebo Responses
Placebo responses occur when patients experience real physiological or psychological improvements after receiving an inert substance, driven by expectations and brain mechanisms. Neuroimaging studies reveal that the brain's reward and pain modulation systems, including the prefrontal cortex and endogenous opioid pathways, are activated during placebo effects. Understanding the science behind placebo responses enhances clinical trial design and therapeutic approaches by leveraging the mind-body connection to maximize treatment outcomes.
Drug vs Placebo: Experimental Design
The experimental design of Drug vs Placebo studies typically involves randomized controlled trials (RCTs), where participants are randomly assigned to either the drug treatment group or the placebo group to minimize bias and ensure reliability. Blinding techniques are employed, often double-blind, so neither participants nor researchers know who receives the active drug, enhancing objectivity in measuring drug efficacy and side effects. Statistical analysis compares outcomes between the two groups, focusing on differences in symptom improvement, adverse effects, and overall treatment impact to establish the drug's therapeutic value over the placebo effect.
Measuring Efficacy: Drug Outcomes vs Placebo
Measuring efficacy in drug trials involves comparing drug outcomes directly against placebo effects to determine true therapeutic benefits. Clinical endpoints, symptom reduction scales, and biomarker changes are analyzed for statistically significant differences between active drug and placebo groups. Robust trial designs use double-blind, randomized controls to minimize bias and isolate the drug's pharmacological impact from psychological placebo influences.
Psychological Mechanisms of Placebo
The drug versus placebo comparison highlights the brain's ability to elicit real physiological responses through expectation and conditioning, key psychological mechanisms behind placebo effects. Neuroimaging studies reveal that placebo-induced analgesia involves activation of endogenous opioid and dopamine pathways, showing how belief modulates pain perception. Understanding these psychological mechanisms deepens insights into treatment outcomes and guides the development of more effective clinical interventions.
Case Studies: Drugs Outperforming Placebo
Clinical case studies consistently demonstrate that active drugs outperform placebos in treating various conditions such as depression, anxiety, and chronic pain by providing measurable symptom relief and improving patient outcomes. For example, selective serotonin reuptake inhibitors (SSRIs) exhibit greater efficacy than placebo in major depressive disorder through modulating neurotransmitter levels, which is supported by randomized controlled trials. These findings underscore the therapeutic benefit and mechanism-specific effects of pharmacological treatments over placebo responses.
Placebo in Modern Medicine and Ethics
Placebo plays a critical role in modern medicine by serving as a control in clinical trials, helping to isolate the actual effects of new drugs from psychological or physiological responses. The ethical use of placebos demands strict adherence to informed consent and transparency to prevent deception and protect patient rights. Placebo-controlled studies are fundamental for validating drug efficacy, yet they must balance scientific rigor with ethical responsibility to prioritize patient welfare.
Factors Influencing the Placebo Response
The placebo response is significantly influenced by factors such as patient expectations, the patient-clinician relationship, and the context of treatment administration. Neurobiological mechanisms, including endogenous opioid release and dopamine pathways, also play a crucial role in modulating placebo effects. Understanding these factors is essential for distinguishing true drug efficacy from placebo responses in clinical trials evaluating drug versus placebo outcomes.
Future Perspectives: Enhancing Drug Trials with Placebos
Future perspectives in drug trials emphasize refining placebo methodologies to improve trial accuracy and ethical standards. Advanced biomarkers and digital monitoring tools are being integrated to better distinguish drug effects from placebo responses, enhancing data reliability. Enhanced placebo designs promise to accelerate drug development by optimizing patient outcomes and minimizing trial biases.
Placebo — Drug Infographic
 libterm.com
libterm.com