Tumor suppressor genes regulate cell growth and prevent uncontrolled cell division, which can lead to cancer. Mutations or inactivation of these genes remove critical growth restraints, allowing tumor development. Discover how understanding tumor suppressor genes can impact your approach to cancer prevention and treatment in the rest of this article.
Table of Comparison
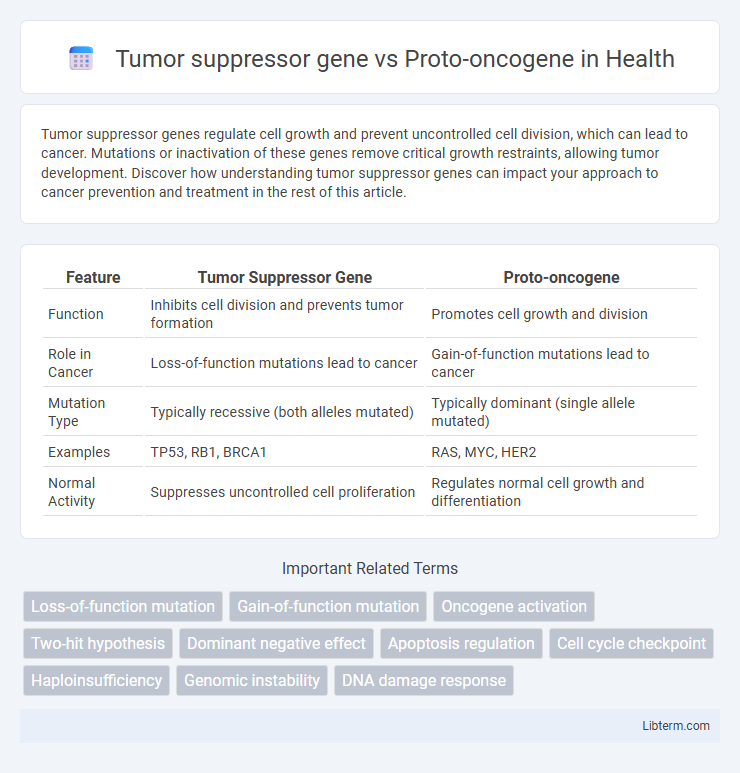
| Feature | Tumor Suppressor Gene | Proto-oncogene |
|---|---|---|
| Function | Inhibits cell division and prevents tumor formation | Promotes cell growth and division |
| Role in Cancer | Loss-of-function mutations lead to cancer | Gain-of-function mutations lead to cancer |
| Mutation Type | Typically recessive (both alleles mutated) | Typically dominant (single allele mutated) |
| Examples | TP53, RB1, BRCA1 | RAS, MYC, HER2 |
| Normal Activity | Suppresses uncontrolled cell proliferation | Regulates normal cell growth and differentiation |
Introduction to Tumor Suppressor Genes and Proto-oncogenes
Tumor suppressor genes regulate cell growth by inhibiting excessive proliferation and maintaining genomic stability, preventing tumor development. Proto-oncogenes encode proteins that promote normal cell division and differentiation, but when mutated, they become oncogenes driving uncontrolled cell growth. The balance between tumor suppressor gene activity and proto-oncogene function is critical for cellular homeostasis and cancer prevention.
Defining Tumor Suppressor Genes
Tumor suppressor genes regulate cell growth by inhibiting uncontrolled cell division and promoting DNA repair or apoptosis, preventing tumor formation. Mutations in these genes lead to loss of function, facilitating cancer progression by allowing cells to proliferate unchecked. Unlike proto-oncogenes, which drive cell division when mutated into oncogenes, tumor suppressor genes act as cellular brakes critical to maintaining genomic integrity.
Understanding Proto-oncogenes
Proto-oncogenes are normal genes that regulate cell growth and division, playing a crucial role in cellular communication and signaling pathways. When mutated or abnormally expressed, proto-oncogenes transform into oncogenes, leading to uncontrolled cell proliferation and contributing to cancer development. Understanding proto-oncogenes involves studying their molecular mechanisms, including gene mutations, amplifications, and translocations, which disrupt normal cellular regulation.
Mechanisms of Tumor Suppressor Gene Action
Tumor suppressor genes regulate cell growth by producing proteins that repair DNA damage, initiate apoptosis, and inhibit cell cycle progression, preventing uncontrolled proliferation. These genes act as critical checkpoints, ensuring damaged or mutated cells do not divide and form tumors. Loss or inactivation of tumor suppressor genes, such as TP53 or RB1, disrupts these mechanisms, leading to increased cancer risk.
Mechanisms of Proto-oncogene Activation
Proto-oncogene activation occurs through mutations, gene amplification, or chromosomal translocations that lead to increased or unregulated protein expression promoting cell proliferation. These genetic alterations convert normal proto-oncogenes into oncogenes, driving oncogenesis by disrupting normal cellular growth controls. Unlike tumor suppressor genes that inhibit cell division, activated proto-oncogenes function as dominant drivers of tumor development through gain-of-function mutations.
Key Differences Between Tumor Suppressor Genes and Proto-oncogenes
Tumor suppressor genes regulate cell growth by inhibiting cell division and promoting apoptosis, functioning as critical brakes to prevent uncontrolled proliferation, whereas proto-oncogenes promote cell growth and division under normal conditions but can become oncogenes if mutated or overexpressed. Mutations in tumor suppressor genes typically result in loss of function, leading to unchecked cell growth, while mutations in proto-oncogenes result in gain of function, driving abnormal cell proliferation. Key examples include TP53 and RB1 as tumor suppressor genes, contrasted with the proto-oncogenes MYC and RAS, each playing distinct roles in cancer development pathways.
Mutations: Loss of Function vs Gain of Function
Tumor suppressor genes undergo loss-of-function mutations that inactivate their ability to regulate cell growth and prevent tumor formation, leading to uncontrolled cell proliferation. Proto-oncogenes experience gain-of-function mutations that convert them into oncogenes, promoting excessive cell division and oncogenesis. The contrasting mutation types--loss of function in tumor suppressor genes and gain of function in proto-oncogenes--are fundamental mechanisms driving cancer development.
Examples of Tumor Suppressor Genes and Proto-oncogenes
Tumor suppressor genes such as TP53, BRCA1, and RB1 play a crucial role in regulating cell growth by preventing uncontrolled proliferation and promoting apoptosis. Proto-oncogenes including MYC, RAS, and HER2 encode proteins that stimulate cell division and differentiation, but when mutated, they become oncogenes that drive cancer progression. The loss of function in tumor suppressor genes or gain of function in proto-oncogenes is a key mechanism in carcinogenesis.
Role in Cancer Development and Progression
Tumor suppressor genes inhibit cell division and promote apoptosis, preventing uncontrolled cell growth, while proto-oncogenes regulate normal cell growth and differentiation but can become oncogenes through mutation, driving cancer development. Mutations or inactivation of tumor suppressor genes like TP53 or RB1 lead to loss of cell cycle control, contributing to tumor progression. Conversely, gain-of-function mutations in proto-oncogenes such as MYC or RAS result in sustained proliferative signaling, accelerating cancer progression and metastasis.
Therapeutic Implications and Future Research Directions
Tumor suppressor genes, such as TP53, inhibit uncontrolled cell growth by inducing cell cycle arrest and apoptosis, making their restoration a promising therapeutic approach through gene therapy and targeted drugs like PARP inhibitors. Proto-oncogenes, including RAS and MYC, drive cancer progression when mutated or overexpressed, with therapies focusing on signaling pathway inhibitors and RNA interference to suppress oncogenic activity. Future research aims to enhance precision medicine by integrating genomic profiling to tailor treatments targeting both tumor suppressor gene reactivation and proto-oncogene inhibition, improving efficacy and minimizing resistance mechanisms.
Tumor suppressor gene Infographic
 libterm.com
libterm.com