Toxicity refers to the degree to which a substance can cause harm to living organisms, affecting their health or environment. Understanding toxicity levels is essential for managing risks in industries such as pharmaceuticals, agriculture, and waste disposal. Explore the rest of this article to discover how toxicity impacts your safety and the environment.
Table of Comparison
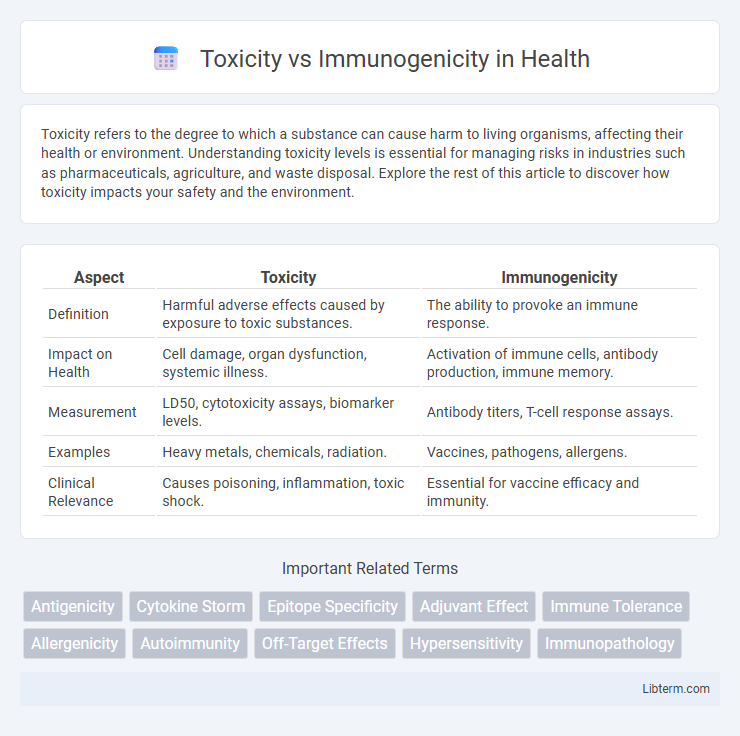
| Aspect | Toxicity | Immunogenicity |
|---|---|---|
| Definition | Harmful adverse effects caused by exposure to toxic substances. | The ability to provoke an immune response. |
| Impact on Health | Cell damage, organ dysfunction, systemic illness. | Activation of immune cells, antibody production, immune memory. |
| Measurement | LD50, cytotoxicity assays, biomarker levels. | Antibody titers, T-cell response assays. |
| Examples | Heavy metals, chemicals, radiation. | Vaccines, pathogens, allergens. |
| Clinical Relevance | Causes poisoning, inflammation, toxic shock. | Essential for vaccine efficacy and immunity. |
Understanding Toxicity and Immunogenicity
Toxicity refers to the degree to which a substance can cause harmful effects to an organism, impacting cellular or systemic functions and leading to adverse reactions. Immunogenicity describes the ability of a substance, such as a drug or vaccine, to provoke an immune response, which can be beneficial for protection or problematic if it triggers immune-related side effects. Understanding toxicity and immunogenicity is crucial in drug development to balance therapeutic efficacy with safety, minimizing adverse immune reactions while ensuring effective treatment outcomes.
Key Differences Between Toxicity and Immunogenicity
Toxicity refers to the degree to which a substance can cause harmful effects on an organism, impacting organs or physiological functions, while immunogenicity describes the ability of a substance, such as a vaccine or therapeutic protein, to provoke an immune response. Key differences include toxicity leading to adverse health effects like organ damage or systemic toxicity, whereas immunogenicity involves activation of the immune system that can be beneficial for vaccines or problematic in biologic drugs due to potential immune reactions. Assessing toxicity focuses on safety profiles and dose-dependent harmful outcomes, whereas evaluating immunogenicity involves measuring antibody production, immune activation, and potential hypersensitivity or autoimmunity risks.
Mechanisms Underlying Toxicity
Toxicity arises from immune system overactivation, causing excessive cytokine release and tissue damage, while immunogenicity refers to the immune response's ability to recognize and react to antigens. Mechanisms underlying toxicity include activation of macrophages, dendritic cells, and T cells leading to cytokine storm, oxidative stress, and apoptosis. Understanding these pathways is crucial for balancing therapeutic efficacy and minimizing adverse immune reactions in drug and vaccine development.
Mechanisms Underlying Immunogenicity
Immunogenicity arises from the immune system's recognition of foreign antigens, leading to activation of adaptive responses involving antigen-presenting cells, T cells, and B cells producing specific antibodies. Mechanisms underlying immunogenicity include structural features such as protein aggregation, glycosylation patterns, and epitope diversity, which influence antigen processing and presentation via major histocompatibility complex (MHC) molecules. These factors dictate the magnitude and specificity of immune activation, differentiating immunogenic responses from non-specific toxicity caused by off-target cellular damage or inflammatory pathways.
Factors Influencing Toxicity in Therapeutics
Factors influencing toxicity in therapeutics include drug dose, administration route, and patient-specific variables such as genetic predisposition, age, and organ function. Metabolic pathways and drug interactions also critically affect the accumulation of toxic metabolites, increasing adverse effects. Understanding these determinants is essential for optimizing therapeutic windows while minimizing immunogenic responses and toxicity.
Determinants of Immunogenic Responses
Determinants of immunogenic responses include antigen structure, dose, and route of administration, which influence the immune system's ability to recognize and react to foreign substances. Protein aggregation, glycosylation patterns, and the presence of pathogen-associated molecular patterns (PAMPs) also play critical roles in modulating immunogenicity. Understanding these factors helps in designing safer biotherapeutics by minimizing toxic reactions while enhancing desired immune activation.
Assessing and Measuring Toxicity
Assessing toxicity involves evaluating adverse biological effects caused by a substance on cellular, tissue, or organism levels, using methods like in vitro cytotoxicity assays, animal models, and clinical biomarkers. Measuring toxicity quantifies parameters such as cytokine release, organ damage markers, and dose-response relationships to identify safe exposure limits. Accurate toxicity assessment is critical in drug development and immunotherapy to minimize adverse reactions while maintaining therapeutic efficacy.
Evaluating Immunogenicity in Drug Development
Evaluating immunogenicity in drug development involves assessing the immune response triggered by a therapeutic agent to predict potential adverse effects and treatment efficacy. Immunogenicity testing includes in vitro assays, animal models, and clinical monitoring to detect anti-drug antibodies and assess their impact on drug safety and effectiveness. Understanding the balance between toxicity and immunogenicity is critical for optimizing biologic drug design and minimizing immune-related complications.
Strategies to Minimize Toxicity and Immunogenicity
Strategies to minimize toxicity and immunogenicity in therapeutics include optimizing molecular design to reduce off-target effects and immune activation by employing humanized or fully human antibodies. Implementing controlled-release formulations and dose escalation protocols can further limit adverse immune responses and systemic toxicity. Advanced screening techniques such as in vitro immunogenicity assays and computational modeling enhance early detection and mitigation of potential immune-related risks.
Clinical Implications: Balancing Safety and Efficacy
Toxicity and immunogenicity directly influence clinical outcomes by determining a treatment's safety and efficacy profiles. Minimizing toxicity is crucial to prevent adverse reactions that can limit therapy tolerability, while managing immunogenicity is essential to avoid immune responses that may reduce drug effectiveness or cause hypersensitivity. Achieving an optimal balance requires rigorous monitoring and personalized dosing strategies to maximize therapeutic benefits while safeguarding patient health.
Toxicity Infographic
 libterm.com
libterm.com