Angiogenesis is the physiological process through which new blood vessels form from pre-existing vessels, playing a crucial role in wound healing, tissue growth, and the body's response to injury. This process is tightly regulated by various growth factors and cellular signals to maintain vascular health and prevent diseases. Explore the rest of the article to understand how angiogenesis impacts your body and its significance in medical research.
Table of Comparison
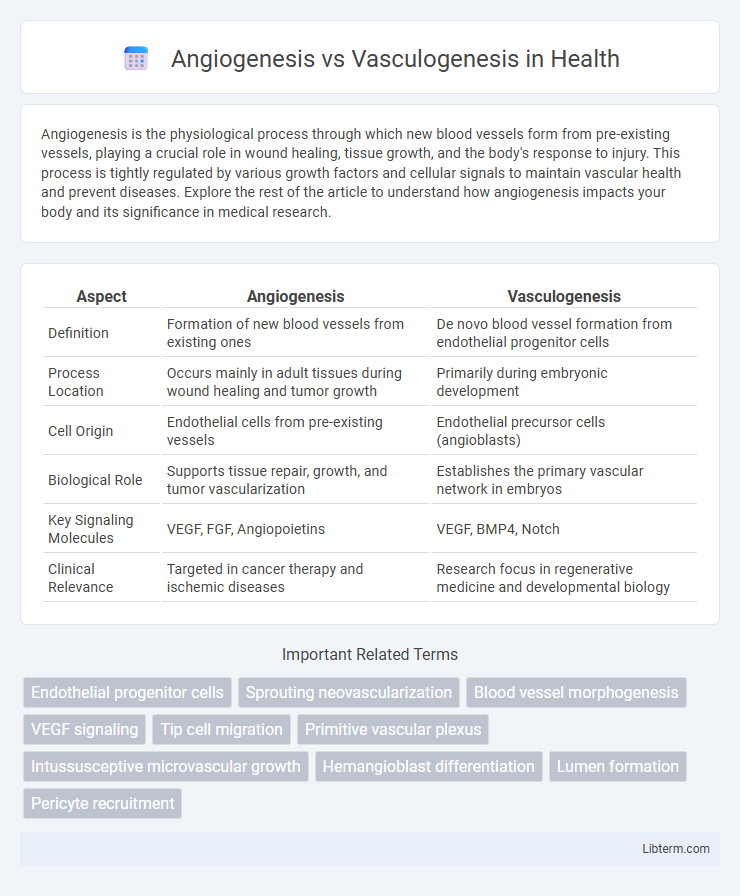
| Aspect | Angiogenesis | Vasculogenesis |
|---|---|---|
| Definition | Formation of new blood vessels from existing ones | De novo blood vessel formation from endothelial progenitor cells |
| Process Location | Occurs mainly in adult tissues during wound healing and tumor growth | Primarily during embryonic development |
| Cell Origin | Endothelial cells from pre-existing vessels | Endothelial precursor cells (angioblasts) |
| Biological Role | Supports tissue repair, growth, and tumor vascularization | Establishes the primary vascular network in embryos |
| Key Signaling Molecules | VEGF, FGF, Angiopoietins | VEGF, BMP4, Notch |
| Clinical Relevance | Targeted in cancer therapy and ischemic diseases | Research focus in regenerative medicine and developmental biology |
Understanding Angiogenesis and Vasculogenesis
Angiogenesis is the physiological process where new blood vessels form from pre-existing vessels, primarily driven by endothelial cell proliferation and migration, essential for wound healing and tumor growth. Vasculogenesis involves the de novo formation of blood vessels from endothelial progenitor cells during embryonic development, establishing the primary vascular network. Understanding the molecular mechanisms and signaling pathways, such as VEGF in both angiogenesis and vasculogenesis, highlights their distinct yet complementary roles in vascular biology.
Historical Background of Blood Vessel Formation
The historical background of blood vessel formation traces back to the 18th century when angiogenesis was first described by John Hunter as the sprouting of new vessels from existing ones. Vasculogenesis, identified later, refers to the de novo formation of blood vessels from endothelial progenitor cells during embryonic development, a concept solidified in the 20th century through advances in embryology and molecular biology. Landmark studies in the 1970s and 1980s elucidated the distinct molecular pathways regulating both angiogenesis and vasculogenesis, shaping current understanding in vascular biology.
Key Molecular Mechanisms in Angiogenesis
Angiogenesis primarily involves the sprouting of new blood vessels from pre-existing vasculature, driven by key molecular mechanisms such as the activation of vascular endothelial growth factor (VEGF) signaling pathways, fibroblast growth factors (FGFs), and the Notch signaling cascade. Matrix metalloproteinases (MMPs) facilitate extracellular matrix remodeling, enabling endothelial cell migration and capillary tube formation. Hypoxia-inducible factor 1-alpha (HIF-1a) plays a critical role in upregulating VEGF expression under low oxygen conditions, orchestrating the angiogenic response.
Core Processes Underlying Vasculogenesis
Vasculogenesis involves the de novo formation of blood vessels from endothelial progenitor cells (EPCs) differentiating into endothelial cells, which then assemble into primitive vascular networks during embryonic development. Core processes underlying vasculogenesis include the migration, proliferation, and differentiation of angioblasts, regulated by molecular signals such as vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF). Unlike angiogenesis, which remodels and extends existing vessels, vasculogenesis establishes the primary vascular plexus essential for subsequent blood flow and tissue perfusion.
Cellular Players: Endothelial Cells in Vessel Formation
Endothelial cells are central to both angiogenesis and vasculogenesis, driving new blood vessel formation through distinct mechanisms. In vasculogenesis, endothelial progenitor cells differentiate and assemble into primitive vascular networks during embryonic development, establishing the initial blood vessel framework. During angiogenesis, mature endothelial cells proliferate, migrate, and sprout from existing vessels to expand and remodel the vascular system in response to physiological needs or tissue repair.
Genetic Regulation of Angiogenesis and Vasculogenesis
Angiogenesis and vasculogenesis are critical processes in blood vessel formation, regulated by distinct genetic pathways. Angiogenesis is primarily driven by VEGF (vascular endothelial growth factor) signaling, with genes such as VEGFA, ANGPT1, and DLL4 playing key roles in endothelial cell proliferation and sprouting. Vasculogenesis involves the differentiation of endothelial progenitor cells regulated by transcription factors like ETV2, KDR (VEGFR2), and NOTCH1, which orchestrate the initial assembly of vascular networks during embryogenesis.
Physiological Roles in Embryonic and Adult Tissues
Angiogenesis primarily facilitates the formation of new blood vessels from existing ones, playing a crucial role in wound healing and the menstrual cycle in adult tissues, while vasculogenesis involves the de novo formation of blood vessels from endothelial progenitor cells, essential during early embryonic development for establishing the primary vascular network. In embryonic tissues, vasculogenesis is indispensable for forming the initial circulatory system, whereas angiogenesis supports vascular remodeling and growth as the embryo develops. In adult physiology, angiogenesis maintains tissue homeostasis and repair, whereas vasculogenesis is limited but can be reactivated in response to ischemic injury or in certain pathological conditions like tumor growth.
Clinical Implications: Diseases Linked to Vascular Formation
Angiogenesis and vasculogenesis play critical roles in the progression of diseases such as cancer, diabetic retinopathy, and ischemic heart conditions by influencing abnormal or insufficient blood vessel formation. Tumor growth and metastasis heavily rely on angiogenesis, making VEGF inhibitors a pivotal therapeutic target in oncology. Vasculogenesis, primarily active during embryonic development, is implicated in congenital vascular malformations and tissue regeneration, which are vital considerations in regenerative medicine and vascular repair strategies.
Therapeutic Approaches Targeting Angiogenesis and Vasculogenesis
Therapeutic approaches targeting angiogenesis primarily involve the use of angiogenesis inhibitors such as bevacizumab, which block vascular endothelial growth factor (VEGF) signaling to restrict tumor blood supply. Vasculogenesis therapies focus on mobilizing and transplanting endothelial progenitor cells (EPCs) to promote new blood vessel formation in ischemic tissues. Combining angiogenesis inhibitors with vasculogenesis-stimulating agents offers potential synergy for treating diseases like cancer and peripheral artery disease by regulating pathological and physiological neovascularization.
Future Perspectives in Vascular Biology Research
Future perspectives in vascular biology research emphasize the integration of advanced imaging and molecular techniques to unravel the distinct mechanisms of angiogenesis and vasculogenesis in tissue regeneration and disease progression. Emerging studies explore targeted therapeutic strategies that selectively modulate endothelial cell behavior to enhance vascular network formation and repair in ischemic conditions and cancer. Innovations in biomaterials and gene editing offer promising avenues for manipulating vascular growth pathways, potentially revolutionizing treatments for chronic wounds, cardiovascular diseases, and tumor angiogenesis.
Angiogenesis Infographic
 libterm.com
libterm.com