Discrete measure assigns positive values to countable sets, often focusing on individual points rather than continuous intervals. It plays a crucial role in probability theory, where probabilities are assigned to distinct outcomes in a sample space. Explore the rest of the article to understand how discrete measures apply in various mathematical and applied contexts.
Table of Comparison
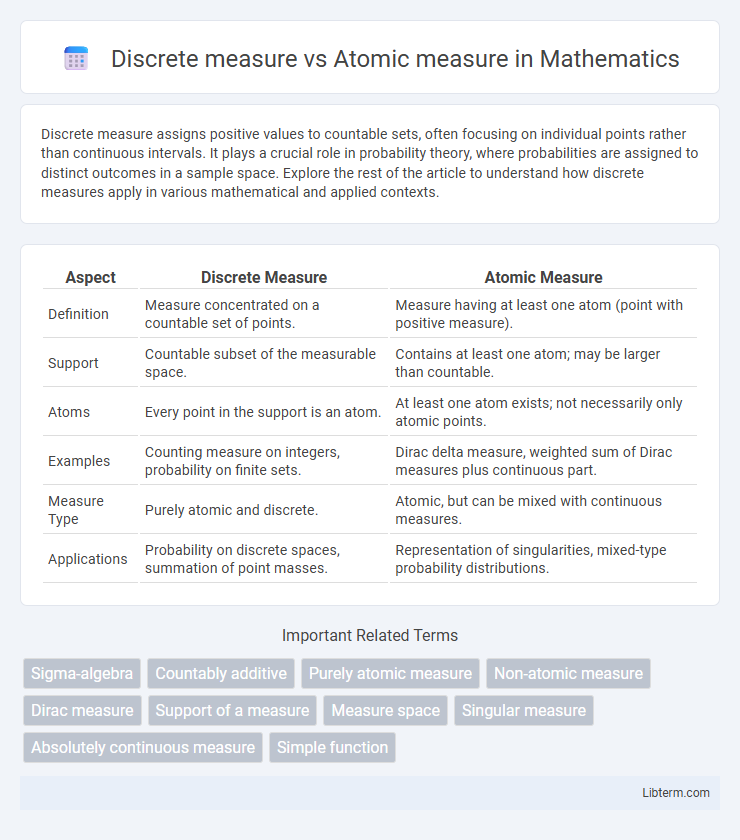
| Aspect | Discrete Measure | Atomic Measure |
|---|---|---|
| Definition | Measure concentrated on a countable set of points. | Measure having at least one atom (point with positive measure). |
| Support | Countable subset of the measurable space. | Contains at least one atom; may be larger than countable. |
| Atoms | Every point in the support is an atom. | At least one atom exists; not necessarily only atomic points. |
| Examples | Counting measure on integers, probability on finite sets. | Dirac delta measure, weighted sum of Dirac measures plus continuous part. |
| Measure Type | Purely atomic and discrete. | Atomic, but can be mixed with continuous measures. |
| Applications | Probability on discrete spaces, summation of point masses. | Representation of singularities, mixed-type probability distributions. |
Introduction to Discrete and Atomic Measures
Discrete measures assign positive measure values to countable sets, often concentrated on isolated points within a measurable space, making them ideal for representing distributions with distinct, separated masses. Atomic measures specifically focus on point masses or atoms, where the measure of any subset containing an atom equals at least the measure of that atom, emphasizing indivisible units of measure. Understanding the distinction between discrete and atomic measures is crucial for applications in probability theory, ergodic theory, and measure-theoretic foundations of analysis.
Defining Discrete Measure: Key Characteristics
A discrete measure is characterized by assigning positive mass exclusively to countable sets of points, making it inherently atomic in nature. Each atom in a discrete measure corresponds to a point with strictly positive measure, ensuring the measure can be represented as a sum of weighted Dirac delta functions. This contrasts with general atomic measures, which may include uncountably many atoms or combinations where atomicity is preserved but discreteness is not guaranteed.
Understanding Atomic Measure: Essential Concepts
An atomic measure is defined by the presence of atoms, or points with positive measure, which distinguish it from a discrete measure that assigns positive measure solely to isolated points in a countable set. In atomic measures, these atoms act as fundamental building blocks, allowing the measure to be decomposed into a sum of Dirac measures concentrated at single points. Understanding atomic measures involves recognizing how each atom captures concentrated mass, essential for applications in probability theory and spectral analysis.
Fundamental Differences Between Discrete and Atomic Measures
Discrete measures assign positive mass exclusively to countable sets, with each point mass potentially varying in size, whereas atomic measures concentrate all mass on individual atoms, each representing a non-divisible unit of measure. The fundamental difference lies in the structure: discrete measures may consist of a countable sum of Dirac delta functions with varying weights, while atomic measures specifically focus on these indivisible atomic masses that cannot be further decomposed. This distinction impacts integration and measure-theoretic properties, as atomic measures emphasize indivisibility and minimal support, contrasting with the potentially more flexible distribution of discrete measures.
Mathematical Formulation of Discrete Measures
Discrete measures are defined on a measurable space by assigning positive weights to countably many points, expressed mathematically as \(\mu = \sum_{i=1}^\infty a_i \delta_{x_i}\), where \(a_i > 0\) are weights and \(\delta_{x_i}\) denotes the Dirac measure at point \(x_i\). Atomic measures identify these points \(x_i\) as atoms where \(\mu(\{x_i\}) = a_i > 0\), ensuring the measure consists entirely of isolated mass concentrations. The mathematical formulation of discrete measures thus involves summing weighted Dirac deltas over a countable set of atoms, contrasting with more general measures that may assign mass over continuous regions.
Structure and Properties of Atomic Measures
Atomic measures consist of point masses concentrated on atoms, which are measurable sets with positive measure that cannot be subdivided into smaller sets of positive measure. Unlike discrete measures that allocate mass exclusively to isolated points, atomic measures can assign mass to atoms that may not be singletons but still retain indivisibility in measure. The structure of atomic measures ensures countable additivity, where the total measure can be written as a sum over atoms, each with a specific positive weight, reflecting their indivisible nature in measure theory.
Examples Illustrating Discrete and Atomic Measures
A discrete measure assigns positive measure to countably many isolated points, such as the counting measure on the integers where each integer has measure one, illustrating a sum over distinct atoms. An atomic measure focuses on measures concentrated on individual atoms, for instance, the Dirac measure d_x which places all mass at a single point x, exemplifying pure atomicity with no measure spread over intervals. These measures highlight how discrete measures encompass countable collections of atoms while atomic measures pinpoint singular atoms with non-zero mass.
Applications in Probability and Real Analysis
Discrete measures assign positive mass to countable sets, commonly applied in probability for modeling distributions of discrete random variables such as Bernoulli or Poisson processes. Atomic measures concentrate mass on individual points called atoms, facilitating the analysis of singular components in measures and enabling decomposition theorems in real analysis. These measures are essential in spectral theory and ergodic theory, where understanding point masses aids in characterizing measures and their integrals.
Advantages and Limitations of Each Measure
Discrete measures clearly identify individual points with positive mass, making them ideal for modeling phenomena concentrated at specific events or locations, such as populations in cities or transaction times. Their advantage lies in straightforward computation and intuitive interpretation, but they lack smoothness and fail to capture continuous variations or distributions. Atomic measures extend discrete measures by focusing on atoms with positive measure, enabling nuanced analysis of point masses; however, they may omit non-atomic components, limiting their effectiveness in representing mixed or purely continuous phenomena.
Summary and Key Takeaways
Discrete measures assign positive mass to countable sets, concentrating measure on isolated points, whereas atomic measures specifically focus on atoms--points with positive measure where no smaller subset has positive measure. Key takeaways include that all atomic measures are discrete but not all discrete measures are purely atomic; discrete measures can contain both atomic and non-atomic parts. Understanding this distinction is crucial in measure theory, especially in probability and integration where the structure of the measure influences the behavior of measurable functions.
Discrete measure Infographic
 libterm.com
libterm.com