Efficient energy use plays a pivotal role in reducing costs and minimizing environmental impact while maintaining comfort and productivity. Innovative technologies and renewable sources are transforming how you generate and consume power, highlighting the importance of sustainable solutions. Explore the rest of this article to discover practical strategies for optimizing your energy consumption and embracing a greener future.
Table of Comparison
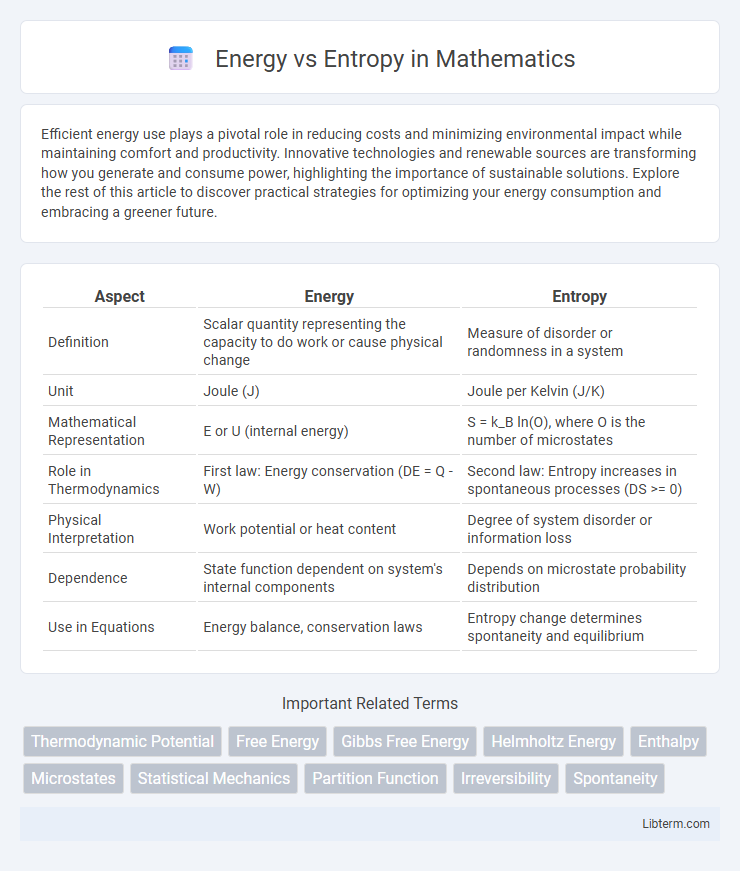
| Aspect | Energy | Entropy |
|---|---|---|
| Definition | Scalar quantity representing the capacity to do work or cause physical change | Measure of disorder or randomness in a system |
| Unit | Joule (J) | Joule per Kelvin (J/K) |
| Mathematical Representation | E or U (internal energy) | S = k_B ln(O), where O is the number of microstates |
| Role in Thermodynamics | First law: Energy conservation (DE = Q - W) | Second law: Entropy increases in spontaneous processes (DS >= 0) |
| Physical Interpretation | Work potential or heat content | Degree of system disorder or information loss |
| Dependence | State function dependent on system's internal components | Depends on microstate probability distribution |
| Use in Equations | Energy balance, conservation laws | Entropy change determines spontaneity and equilibrium |
Understanding Energy and Entropy: Core Concepts
Energy represents the capacity to perform work or induce change, measured in joules (J), and is conserved in isolated systems. Entropy quantifies the degree of disorder or randomness within a system, measured in joules per kelvin (J/K), reflecting the second law of thermodynamics that states entropy tends to increase in spontaneous processes. Understanding the interplay between energy and entropy is essential for analyzing thermodynamic efficiency, chemical reactions, and physical transformations in various scientific fields.
The Laws of Thermodynamics Explained
The First Law of Thermodynamics states that energy cannot be created or destroyed, only transformed, highlighting the conservation of energy within a system. The Second Law introduces entropy, defining it as a measure of disorder, and asserts that entropy in an isolated system always increases, driving spontaneous processes and indicating irreversible energy dispersal. These laws collectively explain energy transfer, transformation, and the natural progression toward thermodynamic equilibrium in physical and chemical systems.
How Energy Flows in Physical Systems
Energy flows in physical systems through processes such as conduction, convection, and radiation, driving changes in the system's state and maintaining dynamic equilibrium. The second law of thermodynamics governs the direction of energy transfer, favoring irreversible dispersion and an increase in entropy. Understanding energy gradients and coupling mechanisms is essential for predicting system behavior and efficiency in natural and engineered environments.
Entropy: The Measure of Disorder
Entropy quantifies the degree of disorder or randomness in a system, reflecting the number of microscopic configurations that correspond to a thermodynamic system's macroscopic state. In thermodynamics, entropy tends to increase over time, driving processes toward equilibrium and illustrating the natural tendency toward chaos and irreversibility. This fundamental principle underpins the second law of thermodynamics, making entropy a crucial metric in fields such as physics, chemistry, and information theory.
The Interplay Between Energy and Entropy
The interplay between energy and entropy governs the direction of natural processes, where energy transformations often lead to an increase in entropy, reflecting the system's move towards disorder and equilibrium. In thermodynamics, systems spontaneously evolve towards states that minimize free energy while maximizing entropy, balancing energy conservation with the universal drive for randomness. This dynamic relationship is fundamental in understanding phenomena such as chemical reactions, phase changes, and biological organization.
Real-World Examples of Energy vs Entropy
Solar panels convert sunlight (energy) into electricity, illustrating energy flow in a practical context. Over time, the efficiency of batteries decreases due to entropy, representing the natural progression toward disorder in energy storage systems. Refrigerators maintain low entropy inside by expelling heat, showcasing energy use to reduce entropy locally while increasing it in the surroundings.
Entropy in Daily Life and Nature
Entropy in daily life manifests through the natural progression of disorder and energy dispersal, evident in processes like ice melting, food spoiling, and the aging of living organisms. In nature, entropy drives irreversible changes such as heat flow from warm to cold bodies, the diffusion of gases in the atmosphere, and the gradual decay of structures over time. This fundamental principle underpins thermodynamic laws, illustrating how energy transformations increase the overall randomness and complexity in environmental systems.
Energy Conservation and Entropy Increase
Energy conservation, governed by the first law of thermodynamics, states that energy cannot be created or destroyed, only transformed or transferred within a system. Entropy, a measure of disorder, invariably increases in isolated systems according to the second law of thermodynamics, reflecting the irreversible nature of energy dispersal. This fundamental relationship highlights that while total energy remains constant, the quality or usable portion of energy decreases as entropy rises.
Technological Applications: Managing Energy and Entropy
Technological applications in energy management increasingly focus on optimizing the balance between energy input and entropy production to improve system efficiency and sustainability. Advanced thermodynamic systems, such as heat engines and refrigerators, utilize entropy reduction strategies to maximize useful work output while minimizing energy losses. Innovations in materials science and information technology further enable precise control of entropy in nanoscale devices, enhancing performance in energy storage, conversion, and computational processes.
The Future: Harnessing Energy, Minimizing Entropy
Advancements in renewable energy technologies promise efficient harnessing of solar, wind, and geothermal power to meet growing global demands. Innovations in energy storage and smart grids optimize system efficiency by reducing entropy and energy losses during transmission and conversion. Future developments focus on maximizing sustainable energy use while minimizing entropy to achieve a resilient and low-carbon energy infrastructure.
Energy Infographic
 libterm.com
libterm.com