Isotopic analysis plays a crucial role in fields like archaeology, geology, and environmental science by tracing the origin and age of materials through variations in isotopes. Understanding these variations helps you unlock detailed information about past climates, migration patterns, and chemical processes. Explore the rest of this article to discover how isotopic techniques reveal hidden insights across diverse disciplines.
Table of Comparison
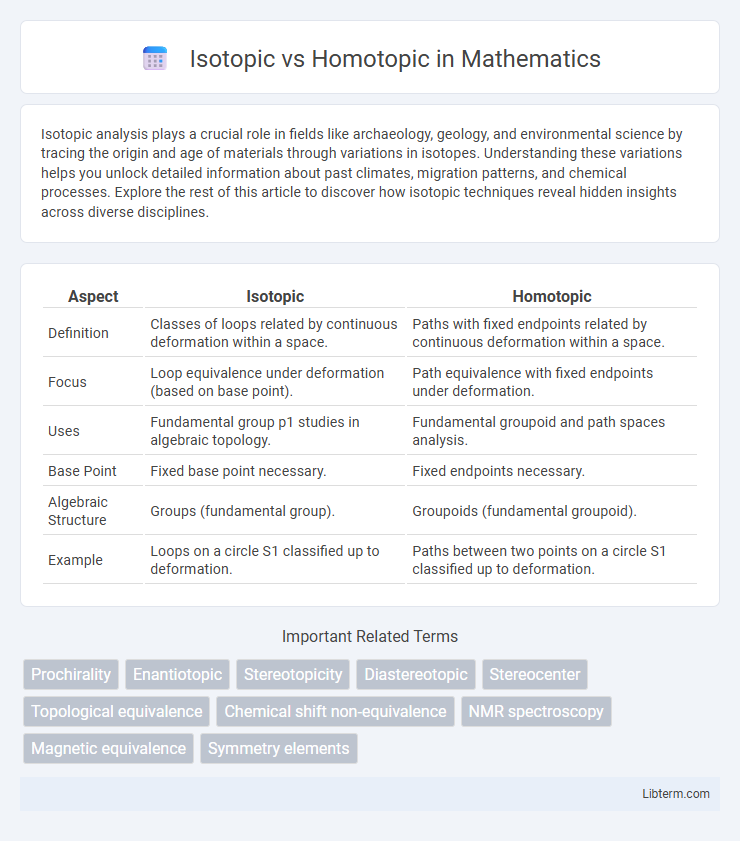
| Aspect | Isotopic | Homotopic |
|---|---|---|
| Definition | Classes of loops related by continuous deformation within a space. | Paths with fixed endpoints related by continuous deformation within a space. |
| Focus | Loop equivalence under deformation (based on base point). | Path equivalence with fixed endpoints under deformation. |
| Uses | Fundamental group p1 studies in algebraic topology. | Fundamental groupoid and path spaces analysis. |
| Base Point | Fixed base point necessary. | Fixed endpoints necessary. |
| Algebraic Structure | Groups (fundamental group). | Groupoids (fundamental groupoid). |
| Example | Loops on a circle S1 classified up to deformation. | Paths between two points on a circle S1 classified up to deformation. |
Introduction to Topological Equivalence
Isotopic and homotopic concepts arise in topology to describe equivalence between continuous functions or embeddings, with isotopy representing a stronger form of equivalence that involves a continuous deformation through homeomorphisms, preserving topological structure at every stage. Homotopy focuses on continuous transformations between maps without requiring invertibility, emphasizing topological spaces' flexibility. Understanding isotopic versus homotopic relations is critical for classifying knots, surfaces, and manifolds in geometric topology, enabling precise characterization of their structural properties.
Defining Isotopic Points and Structures
Isotopic points refer to positions within a molecule that can be substituted by isotopes without altering the molecule's connectivity, often crucial for tracing or labeling in chemical studies. Homotopic points are identical sites in a molecule that are indistinguishable by their chemical environment, resulting in identical chemical shifts in NMR spectroscopy. Defining isotopic structures involves analyzing molecular symmetry and isotopic substitution effects, which is essential for understanding isotopic distribution in complex molecules.
Understanding Homotopic Mapping
Homotopic mapping involves continuous deformation between topological spaces, preserving essential structural properties without tearing or gluing, distinguishing it from isotopic mapping which requires stricter geometric congruence. In homotopy theory, two maps are homotopic if one can be continuously transformed into the other, a concept fundamental for classifying spaces up to continuous deformation. Understanding homotopic mapping enables mathematicians to analyze spaces based on their intrinsic connectivity and shape, rather than rigid geometric form.
Key Differences: Isotopy vs Homotopy
Isotopy refers to a continuous deformation between two embeddings of a manifold in a fixed ambient space, preserving the manifold's structure at every stage, commonly studied in knot theory and topology. Homotopy concerns the continuous deformation between two continuous maps from one topological space to another, focusing on the preservation of homotopical properties rather than embedding details. The key difference lies in isotopy dealing with embeddings and preserving ambient isotopy classes, while homotopy involves more general continuous maps and their homotopy classes.
Mathematical Formalism and Notation
Isotopic and homotopic concepts appear in topology and algebraic structures, with isotopy referring to paths or transformations continuously deformable within a fixed space, often formalized by a parameterized family of homeomorphisms \( f_t: X \to Y \) for \( t \in [0,1] \). Homotopy focuses on mappings between spaces that can be continuously deformed into each other, characterized by a homotopy \( H: X \times [0,1] \to Y \) satisfying \( H(x,0) = f(x) \) and \( H(x,1) = g(x) \) for continuous functions \( f, g: X \to Y \). Notation explicitly distinguishes isotopy as a stricter relation implying homeomorphic deformation respecting ambient space structure, whereas homotopy entails broader continuous deformations between functions or spaces without preservation of embedding.
Examples of Isotopic Transformations
Isotopic transformations involve the substitution of atoms by their isotopes, maintaining the same chemical element but differing in neutron number, such as replacing hydrogen (1H) with deuterium (2H) in chemical reactions. An example includes the kinetic isotope effect observed when the rate of a reaction changes due to the substitution of protium with deuterium, affecting bond vibration frequencies. Another example is the use of carbon-14 (14C) in radiolabeling experiments to trace metabolic pathways without altering the molecule's chemical properties.
Illustrations of Homotopic Equivalence
Homotopic equivalence in topology occurs when two continuous functions between topological spaces can be continuously deformed into each other, preserving fundamental properties like connectedness and holes. Illustrations of homotopic equivalence include examples like the deformation of a circle into an ellipse without tearing or gluing, and the contraction of a disk to a point, demonstrating spaces with the same homotopy type. Isotopic equivalence, in contrast, requires a stronger condition involving ambient isotopies, ensuring one embedding can be transformed into another within the same ambient space.
Practical Applications in Mathematics and Physics
Isotopic and homotopic concepts play crucial roles in topology and algebraic geometry, with isotopy referring to continuous deformations of embeddings preserving topological properties, while homotopy concerns continuous transformations of functions between spaces. In physics, isotopy underpins studies of knot theory in particle physics and the analysis of braiding statistics in quantum computing, whereas homotopy is instrumental in classifying defects in condensed matter systems and understanding gauge symmetries in field theory. Mathematical applications leverage isotopy to analyze knot invariants and spatial transformations, and homotopy to investigate fundamental group structures and topological equivalences in complex manifolds.
Common Misconceptions Explained
Isotopic atoms differ by the number of neutrons in their nuclei, affecting atomic mass but not chemical properties, while homotopic atoms are indistinguishable in their chemical environment and behavior. A common misconception is that isotopic substitution always changes chemical reactivity, but isotopes typically exhibit similar reactivity due to identical electronic structures. Homotopic atoms produce identical NMR signals, whereas isotopic substitution can cause slight shifts, clarifying their distinct roles in spectroscopic analysis.
Conclusion: Choosing Between Isotopic and Homotopic Approaches
Choosing between isotopic and homotopic approaches depends on the specific chemical context and the desired level of distinction in molecular labeling. Isotopic methods offer precise differentiation of atoms with identical chemical environments using isotopes, while homotopic approaches identify equivalent atoms indistinguishable by isotopic substitution. Understanding the molecular symmetry and experimental goals guides the optimal selection between isotopic labeling for detailed mechanistic studies and homotopic analysis for simplifying molecular equivalence in structural characterization.
Isotopic Infographic
 libterm.com
libterm.com