Orthoclase is a common feldspar mineral essential in the formation of granite and other igneous rocks, characterized by its distinctive pink, white, or cream color and monoclinic crystal structure. It plays a crucial role in ceramics, glass-making, and as a gemstone known as moonstone for its shimmering effect called adularescence. To explore the diverse properties and uses of orthoclase, continue reading the full article.
Table of Comparison
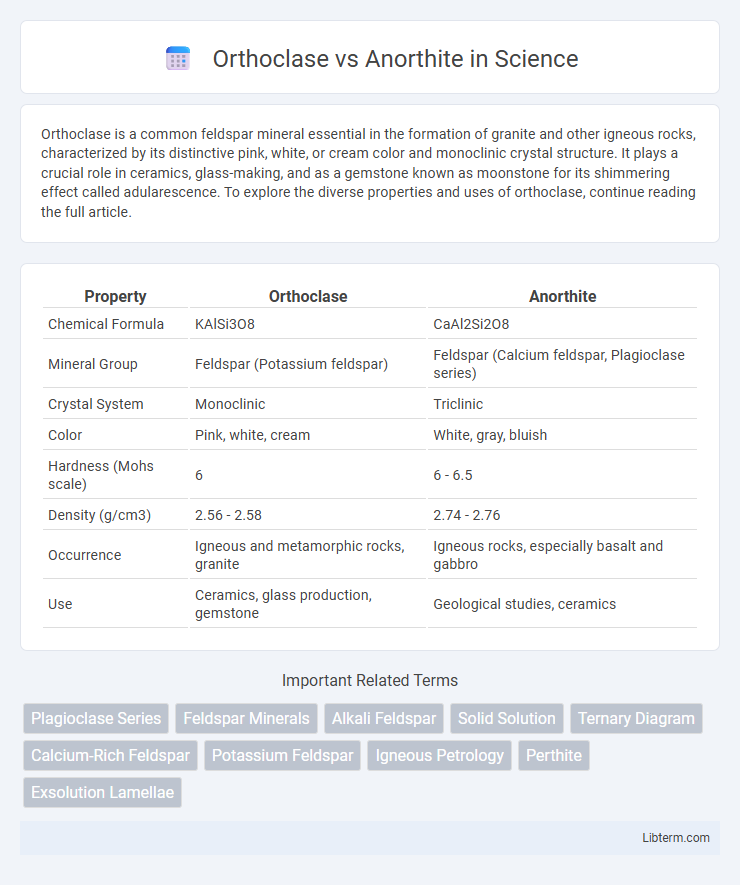
| Property | Orthoclase | Anorthite |
|---|---|---|
| Chemical Formula | KAlSi3O8 | CaAl2Si2O8 |
| Mineral Group | Feldspar (Potassium feldspar) | Feldspar (Calcium feldspar, Plagioclase series) |
| Crystal System | Monoclinic | Triclinic |
| Color | Pink, white, cream | White, gray, bluish |
| Hardness (Mohs scale) | 6 | 6 - 6.5 |
| Density (g/cm3) | 2.56 - 2.58 | 2.74 - 2.76 |
| Occurrence | Igneous and metamorphic rocks, granite | Igneous rocks, especially basalt and gabbro |
| Use | Ceramics, glass production, gemstone | Geological studies, ceramics |
Introduction to Orthoclase and Anorthite
Orthoclase and anorthite are both feldspar minerals, essential components in the Earth's crust with distinct chemical compositions; orthoclase is a potassium aluminum silicate (KAlSi3O8), while anorthite is a calcium aluminum silicate (CaAl2Si2O8). Orthoclase commonly appears in granitic and pegmatitic rocks, playing a critical role in rock formation and weathering processes. Anorthite is predominantly found in mafic igneous rocks and is key to understanding crustal differentiation through its high calcium content.
Mineral Classification and Chemical Composition
Orthoclase and anorthite belong to the feldspar mineral group, classified specifically under potassium feldspar and calcium plagioclase feldspar, respectively. Orthoclase exhibits a chemical composition dominated by potassium aluminum silicate (KAlSi3O8), whereas anorthite is characterized by calcium aluminum silicate (CaAl2Si2O8). The distinct chemical makeup defines their crystallographic structures and influences their physical properties within igneous and metamorphic rocks.
Crystal Structure Differences
Orthoclase and Anorthite both belong to the feldspar mineral group but exhibit distinct crystal structures due to their differing chemical compositions. Orthoclase, a potassium aluminum silicate, crystallizes in the monoclinic system with a symmetry that results in well-formed prismatic crystals. Anorthite, a calcium aluminum silicate, crystallizes in the triclinic system, producing more complex and less symmetrical crystal forms, which leads to variations in optical and physical properties between the two minerals.
Physical Properties Comparison
Orthoclase and Anorthite differ significantly in physical properties, with orthoclase exhibiting a Mohs hardness of 6 and anorthite rated slightly higher at 6.5 to 7. Orthoclase has a specific gravity of about 2.56 to 2.58, whereas anorthite's specific gravity ranges from 2.75 to 2.85, indicating greater density. Both minerals display a vitreous luster, but orthoclase typically shows a white to pink hue, while anorthite commonly appears in shades of white to gray or green.
Geological Occurrence and Formation
Orthoclase, a potassium-rich feldspar, commonly forms in granitic and pegmatitic igneous rocks, crystallizing from silica-rich magmas during the late stages of magma cooling. Anorthite, a calcium-rich plagioclase feldspar, primarily occurs in mafic and ultramafic igneous rocks like gabbros and basalts, often crystallizing early from magnesium- and calcium-rich magmas. Both minerals are integral to igneous petrology, with orthoclase prevalent in continental crust and anorthite more abundant in oceanic crust settings.
Common Uses and Applications
Orthoclase, a potassium-rich feldspar, is widely used in the manufacture of ceramics, glass, and porcelain due to its high melting point and chemical stability. Anorthite, a calcium-rich plagioclase feldspar, finds common applications in the production of ceramics, glass, and as a flux in foundries to lower melting temperatures. Both minerals play crucial roles in industrial processes, with orthoclase preferred for its alkali content and anorthite valued for its calcium content and melting properties.
Visual Identification and Diagnostic Features
Orthoclase and anorthite are feldspar minerals distinguishable by their color and crystal habit; orthoclase typically exhibits a pink to white hue with a monoclinic crystal system, while anorthite shows a white to gray or bluish tint with a triclinic crystal system. Orthoclase features two cleavage planes at nearly right angles and a glassy luster, whereas anorthite displays cleavage angles of about 93deg and 87deg, often accompanied by a slightly duller or pearly luster. Diagnostic features include orthoclase's characteristic tartan twinning visible under polarized light and anorthite's frequent simple lamellar twinning, aiding in their identification in hand specimens and thin sections.
Weathering and Stability in Nature
Orthoclase, a potassium-rich feldspar, weathers slowly in natural environments due to its relatively stable crystal structure, often altering into kaolinite through hydrolysis. Anorthite, a calcium-rich plagioclase feldspar, is more susceptible to chemical weathering, dissolving faster in acidic conditions and commonly transforming into clay minerals and calcite. The stability differences influence soil composition and mineral availability, with orthoclase contributing to longer-lasting mineral residues compared to the more rapidly decomposing anorthite.
Economic and Industrial Importance
Orthoclase, a potassium-rich feldspar, is crucial in the glass and ceramics industries due to its high potassium content, which improves product durability and color. Anorthite, a calcium-rich feldspar, is economically significant in the manufacture of ceramics and glass, enhancing thermal and chemical stability. Both minerals serve as vital raw materials in producing tiles, glassware, and specialized ceramic products, driving their industrial demand.
Key Distinctions and Summary Table
Orthoclase and Anorthite are both feldspar minerals but differ significantly in chemical composition and crystallography; Orthoclase is a potassium-rich feldspar (KAlSi3O8) while Anorthite is calcium-rich (CaAl2Si2O8). Orthoclase typically forms in igneous rocks like granite with a monoclinic crystal system, whereas Anorthite is prevalent in mafic rocks and follows a triclinic crystal system. Key distinctions include their differing refractive indices, hardness (Orthoclase 6, Anorthite 6-6.5), and specific gravity (Orthoclase ~2.56, Anorthite ~2.75), summarized as follows: | Property | Orthoclase (KAlSi3O8) | Anorthite (CaAl2Si2O8) | |--------------------|-----------------------|-----------------------| | Crystal System | Monoclinic | Triclinic | | Chemical Composition| Potassium Aluminum Silicate | Calcium Aluminum Silicate| | Mohs Hardness | 6 | 6 - 6.5 | | Specific Gravity | ~2.56 | ~2.75 | | Common Occurrence | Igneous rocks (granites) | Mafic igneous rocks |
Orthoclase Infographic
 libterm.com
libterm.com