Isothermal processes occur at a constant temperature throughout the entire transformation, ensuring thermal equilibrium with the surroundings. These processes are essential in thermodynamics, often found in systems like ideal gases undergoing slow compression or expansion to maintain temperature stability. Explore the article to understand how isothermal conditions impact energy exchange and system behavior.
Table of Comparison
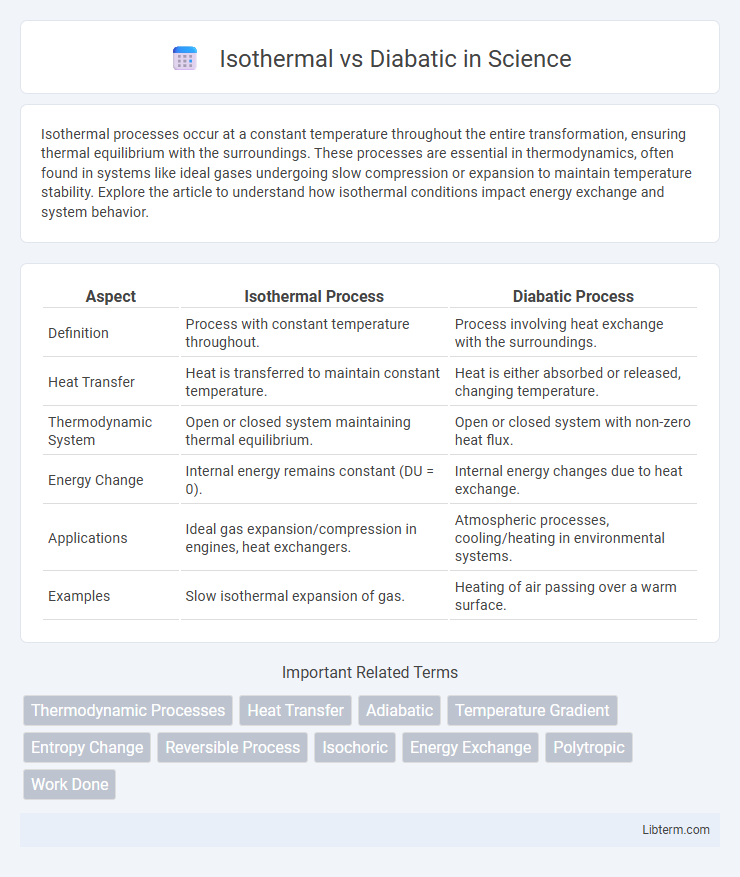
| Aspect | Isothermal Process | Diabatic Process |
|---|---|---|
| Definition | Process with constant temperature throughout. | Process involving heat exchange with the surroundings. |
| Heat Transfer | Heat is transferred to maintain constant temperature. | Heat is either absorbed or released, changing temperature. |
| Thermodynamic System | Open or closed system maintaining thermal equilibrium. | Open or closed system with non-zero heat flux. |
| Energy Change | Internal energy remains constant (DU = 0). | Internal energy changes due to heat exchange. |
| Applications | Ideal gas expansion/compression in engines, heat exchangers. | Atmospheric processes, cooling/heating in environmental systems. |
| Examples | Slow isothermal expansion of gas. | Heating of air passing over a warm surface. |
Introduction to Isothermal and Diabatic Processes
Isothermal processes maintain a constant temperature by allowing heat exchange between the system and its surroundings, often observed in phase changes or ideal gas compression at slow rates. Diabatic processes involve heat transfer where the system's temperature changes due to heat gain or loss, commonly seen in atmospheric phenomena and heat exchangers. Understanding the distinction between isothermal and diabatic processes is crucial for thermodynamics applications, including engine cycles and environmental modeling.
Defining Isothermal Processes
Isothermal processes are characterized by constant temperature throughout the system, ensuring that any heat added or removed is used to perform work without changing the system's internal energy. This contrasts with diabatic processes, where heat exchange causes temperature variations in the system. Understanding isothermal behavior is crucial in thermodynamics, particularly in ideal gas transformations and applications like heat engines and refrigeration cycles.
Defining Diabatic Processes
Diabatic processes involve heat exchange between a system and its surroundings, altering the system's internal energy during thermodynamic transformations. Unlike isothermal processes, which maintain a constant temperature through perfect heat transfer, diabatic processes result in temperature changes due to energy transfer via conduction, convection, or radiation. This distinction is crucial in fields like meteorology and engineering, where understanding heat flux impacts system behavior and energy efficiency.
Key Differences Between Isothermal and Diabatic
Isothermal processes maintain a constant temperature throughout the system, often involving heat exchange with the surroundings to balance internal energy changes. Diabatic processes, in contrast, involve heat transfer across the system boundaries, causing temperature variations within the system. Key differences include the temperature constancy in isothermal conditions versus temperature changes in diabatic conditions, and the role of heat transfer--heat is added or removed in diabatic processes, while isothermal processes rely on heat exchange to sustain equilibrium without temperature fluctuation.
Practical Examples of Isothermal Processes
Isothermal processes maintain a constant temperature throughout, often demonstrated in practical examples such as the slow compression of a gas in a piston where heat exchange with the surroundings prevents temperature change. Industrial applications include the liquefaction of gases and certain refrigeration cycles, where isothermal conditions enable efficient phase changes without temperature fluctuations. These processes rely on controlled heat transfer to maintain thermal equilibrium, distinguishing them from diabatic processes that involve temperature changes due to heat addition or removal.
Practical Examples of Diabatic Processes
Diabatic processes involve heat exchange with the surroundings, exemplified in refrigeration cycles where heat is removed from the cold reservoir to the environment, and in weather systems where heat transfer affects air mass temperature changes. In industrial applications, heat exchangers demonstrate diabatic behavior by transferring thermal energy between fluids at different temperatures. These processes contrast with isothermal ones, where temperature remains constant despite work or heat flows.
Thermodynamic Implications of Each Process
Isothermal processes maintain constant temperature, allowing heat transfer to balance work done, resulting in no changes to internal energy and maximizing efficiency in ideal gas systems. Diabatic processes involve heat exchange that alters system temperature, causing variations in internal energy and entropy, typically reducing efficiency compared to isothermal conditions. Thermodynamic implications of isothermal processes favor energy conservation and reversible work, whereas diabatic changes introduce irreversibility and thermodynamic losses.
Applications in Engineering and Science
Isothermal processes, where temperature remains constant, are crucial in chemical reactors and heat exchangers for maintaining stable reaction conditions and efficient thermal management. Diabatic processes involve heat transfer with surroundings, commonly applied in refrigeration cycles, combustion engines, and atmospheric science to model energy exchange accurately. Engineers and scientists leverage these concepts to optimize system efficiency, control thermodynamic properties, and predict behavior in thermal systems.
Advantages and Limitations of Both Processes
Isothermal processes maintain constant temperature, enabling efficient heat exchange and minimal energy loss, which suits chemical reactions requiring stable thermal conditions; however, they often demand complex temperature control systems and slower reaction rates. Diabatic processes involve temperature changes, offering faster reactions and flexibility in energy input but can lead to inefficiencies due to heat loss and potential thermal degradation of materials. Choosing between isothermal and diabatic methods depends on the need for temperature stability versus reaction speed and energy efficiency in industrial and laboratory settings.
Isothermal vs Diabatic: Which to Choose?
Isothermal processes maintain a constant temperature by allowing heat exchange with the surroundings, making them ideal for applications requiring thermal stability and energy efficiency. Diabatic processes involve heat transfer that alters the system's temperature, suitable for scenarios requiring controlled temperature changes or heat addition/removal. Choosing between isothermal and diabatic depends on whether temperature constancy or temperature variation is crucial for the specific thermodynamic application.
Isothermal Infographic
 libterm.com
libterm.com