Radioluminescence occurs when materials emit light as a result of exposure to ionizing radiation, often useful in low-light or no-light environments. This phenomenon is applied in various fields, including medical imaging, nuclear safety, and watch dials, where continuous illumination without external power is critical. Discover how radioluminescence works and explore its practical applications in your daily life by reading the full article.
Table of Comparison
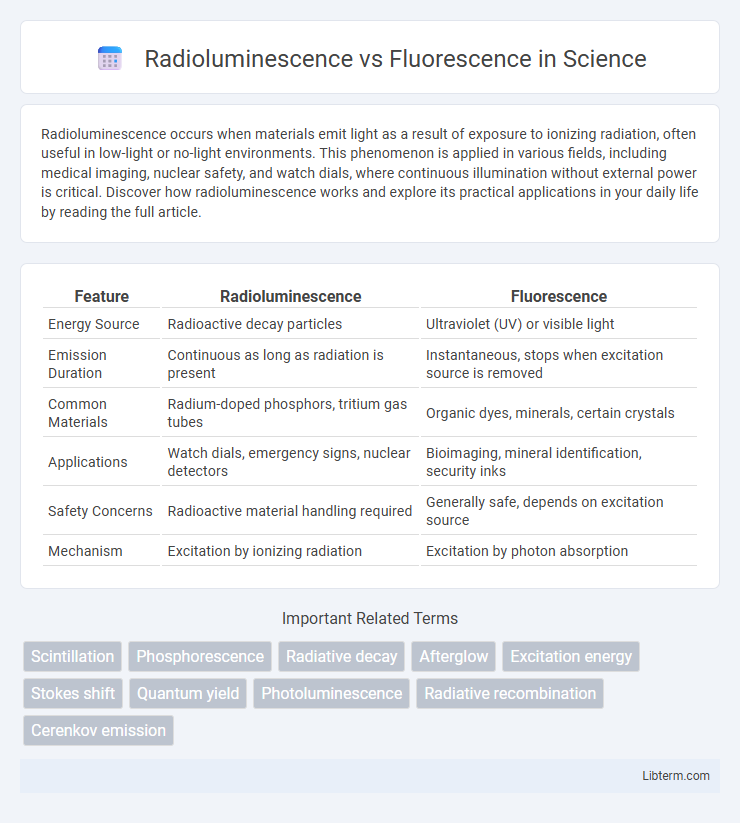
| Feature | Radioluminescence | Fluorescence |
|---|---|---|
| Energy Source | Radioactive decay particles | Ultraviolet (UV) or visible light |
| Emission Duration | Continuous as long as radiation is present | Instantaneous, stops when excitation source is removed |
| Common Materials | Radium-doped phosphors, tritium gas tubes | Organic dyes, minerals, certain crystals |
| Applications | Watch dials, emergency signs, nuclear detectors | Bioimaging, mineral identification, security inks |
| Safety Concerns | Radioactive material handling required | Generally safe, depends on excitation source |
| Mechanism | Excitation by ionizing radiation | Excitation by photon absorption |
Introduction to Radioluminescence and Fluorescence
Radioluminescence occurs when materials emit light upon exposure to ionizing radiation, such as alpha, beta, or gamma particles, making it essential in radiation detection and nuclear imaging. Fluorescence involves the absorption of photons by a substance, followed by the emission of light at a longer wavelength, commonly used in biochemical assays and optical imaging. Both phenomena involve electronic excitation and light emission but differ fundamentally in their excitation sources and applications within scientific and medical fields.
Fundamental Principles of Radioluminescence
Radioluminescence occurs when ionizing radiation interacts with a phosphor material, causing it to emit light through excitation and subsequent photon release. Unlike fluorescence, which relies on ultraviolet or visible light excitation, radioluminescence is powered by alpha, beta, or gamma radiation that excites electrons to higher energy states. The fundamental principle involves energy transfer from radioactive decay particles to the luminescent centers in the phosphor, resulting in continuous light emission independent of external light sources.
Mechanisms Underlying Fluorescence
Fluorescence occurs when molecules absorb photons, exciting electrons to higher energy states, which then rapidly return to their ground state by emitting light of a longer wavelength. This process involves electronic transitions within the molecule's singlet states and typically lasts nanoseconds. Radioluminescence, in contrast, results from the interaction of ionizing radiation with materials, causing electron excitation through energetic particles rather than photon absorption.
Key Differences Between Radioluminescence and Fluorescence
Radioluminescence occurs when materials emit light as a result of exposure to ionizing radiation, whereas fluorescence is triggered by the absorption of ultraviolet or visible light. Radioluminescent substances often involve radioactive isotopes like tritium or radium, emitting light continuously without an external light source, while fluorescent materials only emit light while being excited by an external source and cease immediately afterward. The decay processes differ, with radioluminescence relying on radioactive decay and fluorescence involving electronic excitation and emission transitions within molecules.
Common Materials Exhibiting Radioluminescence
Common materials exhibiting radioluminescence include zinc sulfide doped with copper or silver, strontium aluminate, and certain phosphor compounds integrated with radioactive isotopes like tritium or promethium. These substances emit light when exposed to ionizing radiation, differentiating radioluminescence from fluorescence, which occurs under ultraviolet or visible light excitation. Radioluminescent materials are widely utilized in applications such as emergency lighting, watch dials, and radiation detection devices due to their self-sustained emission properties.
Typical Fluorescent Materials and Their Properties
Typical fluorescent materials include organic compounds like fluorescein, rhodamine, and quinine sulfate, renowned for their high quantum yields and distinct emission spectra within the visible range. Inorganic phosphors such as zinc sulfide doped with copper or manganese exhibit durable luminescent properties and are commonly utilized in lighting and display technologies. These materials absorb ultraviolet or visible light and emit fluorescence rapidly, with lifetimes typically in the nanosecond to microsecond range, contrasting with the longer persistence seen in radioluminescent sources.
Real-World Applications of Radioluminescence
Radioluminescence, the emission of light from a material exposed to ionizing radiation, is widely used in real-world applications such as emergency exit signs, wristwatches, and military instrumentation for nighttime visibility without external power sources. Unlike fluorescence, which requires ultraviolet or visible light excitation, radioluminescent materials maintain consistent glow through radioactive decay, making them ideal for long-lasting, low-maintenance illumination. Key substances like tritium and promethium-147 are commonly embedded in safety equipment and aerospace instruments, ensuring reliable performance in low-light or no-light environments.
Fluorescence in Modern Scientific and Medical Fields
Fluorescence plays a crucial role in modern scientific and medical fields by enabling highly sensitive detection and imaging of biomolecules, cells, and tissues through the emission of light upon excitation by specific wavelengths. Techniques such as fluorescence microscopy, flow cytometry, and fluorescent tagging facilitate detailed visualization and analysis in diagnostics, molecular biology, and pharmaceutical research. Its advantages over radioluminescence include faster response times, safer operational conditions, and the ability to use a wide range of fluorophores for multiplexed and real-time applications.
Advantages and Limitations of Each Phenomenon
Radioluminescence offers the advantage of continuous light emission without the need for external excitation, making it ideal for long-lasting, low-maintenance lighting applications; however, its dependence on radioactive materials poses safety and regulatory limitations. Fluorescence provides rapid, high-intensity light emission when excited by ultraviolet or visible light, which is advantageous for dynamic imaging and sensing technologies, but its emission ceases almost immediately after the excitation source is removed, limiting sustained illumination. Both phenomena have niche uses driven by their emission mechanisms, with radioluminescence favored in persistent lighting and fluorescence preferred in applications requiring quick, reversible light responses.
Future Trends in Luminescence-Based Technologies
Future trends in luminescence-based technologies emphasize the integration of radioluminescence and fluorescence for enhanced sensitivity in medical imaging, environmental monitoring, and security applications. Advances in nanomaterials and quantum dot engineering enable longer-lasting and more efficient luminescent signals under diverse conditions, pushing the boundaries of real-time and deep-tissue visualization. The development of hybrid luminescent systems combining radioactive isotopes and fluorescent probes is expected to revolutionize bioimaging techniques with improved resolution and minimized toxicity.
Radioluminescence Infographic
 libterm.com
libterm.com