Condensation is the process where water vapor in the air cools and changes into liquid droplets, often forming on surfaces like windows or metal. This phenomenon plays a crucial role in the water cycle and impacts everyday environments, from foggy mornings to the formation of dew. Discover how understanding condensation can help you manage moisture and improve comfort in your living space by reading the rest of the article.
Table of Comparison
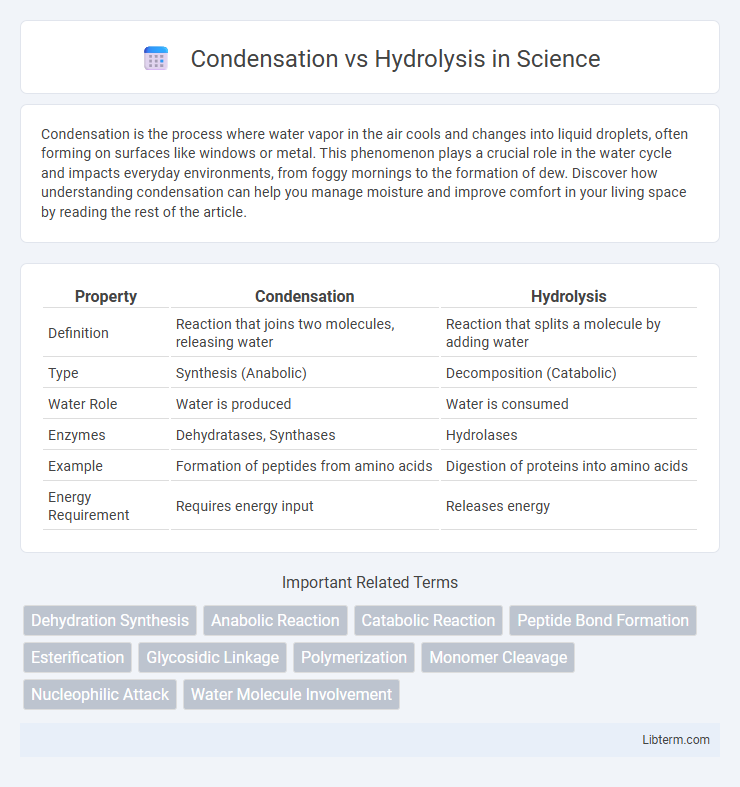
| Property | Condensation | Hydrolysis |
|---|---|---|
| Definition | Reaction that joins two molecules, releasing water | Reaction that splits a molecule by adding water |
| Type | Synthesis (Anabolic) | Decomposition (Catabolic) |
| Water Role | Water is produced | Water is consumed |
| Enzymes | Dehydratases, Synthases | Hydrolases |
| Example | Formation of peptides from amino acids | Digestion of proteins into amino acids |
| Energy Requirement | Requires energy input | Releases energy |
Introduction to Condensation and Hydrolysis
Condensation is a chemical reaction where two molecules combine to form a larger molecule, accompanied by the loss of a small molecule such as water. Hydrolysis is the reverse process, involving the breakdown of a compound through the addition of water, splitting it into smaller units. These reactions are fundamental in biochemical processes like polymer formation and degradation.
Defining Condensation Reactions
Condensation reactions involve the joining of two molecules to form a larger compound while releasing a small molecule, typically water, as a byproduct. These reactions are fundamental in forming complex biological macromolecules such as proteins, nucleic acids, and polysaccharides by linking monomers together. Condensation is the reverse process of hydrolysis, which breaks down these larger molecules by adding water.
Understanding Hydrolysis Reactions
Hydrolysis reactions involve the breakdown of molecules through the addition of water, reversing condensation reactions that form bonds by removing water. These reactions play a crucial role in digestion, where enzymes catalyze the hydrolysis of polymers like proteins, carbohydrates, and lipids into their monomer units. Understanding hydrolysis is essential for biochemical processes such as ATP breakdown and nucleic acid metabolism.
Key Differences Between Condensation and Hydrolysis
Condensation reactions involve the joining of two molecules with the release of a water molecule, commonly forming polymers or larger molecules from monomers. Hydrolysis is the enzymatic or chemical process that breaks down polymers into monomers by adding water, effectively reversing condensation reactions. Key differences include condensation building complex molecules and removing water, while hydrolysis breaks bonds using water, essential in metabolism and digestion.
Biological Importance of Condensation
Condensation reactions play a crucial role in biology by enabling the synthesis of complex macromolecules such as proteins, nucleic acids, and polysaccharides through the formation of covalent bonds and the release of water molecules. These reactions facilitate the assembly of amino acids into polypeptides, nucleotides into DNA or RNA strands, and monosaccharides into polysaccharides, which are essential for cellular structure and function. The efficiency of condensation reactions directly impacts biomolecular synthesis and energy storage in living organisms.
Biological Significance of Hydrolysis
Hydrolysis is crucial in biological systems as it breaks down complex molecules like carbohydrates, proteins, and lipids into their monomers, enabling cellular absorption and energy release. This enzymatic process facilitates digestion, nutrient assimilation, and metabolic regulation by cleaving chemical bonds with water molecules. The biological significance of hydrolysis lies in maintaining homeostasis, supporting cellular respiration, and enabling biochemical pathways essential for life.
Role in Biomolecule Synthesis and Breakdown
Condensation reactions play a crucial role in biomolecule synthesis by forming covalent bonds between monomers, releasing water molecules as byproducts, which leads to the creation of complex macromolecules such as proteins, nucleic acids, and polysaccharides. Hydrolysis reactions facilitate biomolecule breakdown by cleaving these covalent bonds with the addition of water, effectively reversing condensation and enabling the recycling of monomers for cellular processes. Enzymes like polymerases drive condensation during synthesis, while hydrolases catalyze hydrolysis, maintaining metabolic balance and energy homeostasis in biological systems.
Common Examples in Biochemistry
Condensation and hydrolysis are fundamental biochemical reactions involving the formation and breakdown of macromolecules, respectively. Common examples of condensation include the synthesis of proteins through peptide bond formation between amino acids and the creation of polysaccharides like starch via glycosidic bonds. Hydrolysis reactions occur during the digestion of proteins by proteases breaking peptide bonds and the breakdown of polysaccharides into monosaccharides by enzymes such as amylase.
Factors Influencing Condensation and Hydrolysis
Temperature, pH, and enzyme presence significantly influence condensation and hydrolysis reactions, with higher temperatures generally accelerating both processes by increasing molecular movement. Water concentration plays a critical role, as condensation requires removal of water molecules to form bonds, while hydrolysis depends on abundant water to break those bonds. Catalysts such as acids, bases, or specific enzymes like hydrolases can dramatically alter reaction rates and selectivity during condensation and hydrolysis.
Conclusion: Condensation vs Hydrolysis in Biological Systems
Condensation and hydrolysis are fundamental biochemical reactions that work in tandem to regulate molecular synthesis and degradation within biological systems. Condensation reactions form complex molecules such as proteins and polysaccharides by releasing water, while hydrolysis reactions break down these macromolecules by consuming water. This dynamic balance between condensation and hydrolysis ensures cellular function, energy management, and the maintenance of metabolic homeostasis.
Condensation Infographic
 libterm.com
libterm.com