Phagocytosis is a critical immune process where specialized cells engulf and digest harmful pathogens and debris to protect your body from infections. This cellular mechanism involves the recognition, ingestion, and breakdown of foreign particles, playing a vital role in maintaining health. Explore the rest of the article to understand how phagocytosis supports your immune defense and promotes recovery.
Table of Comparison
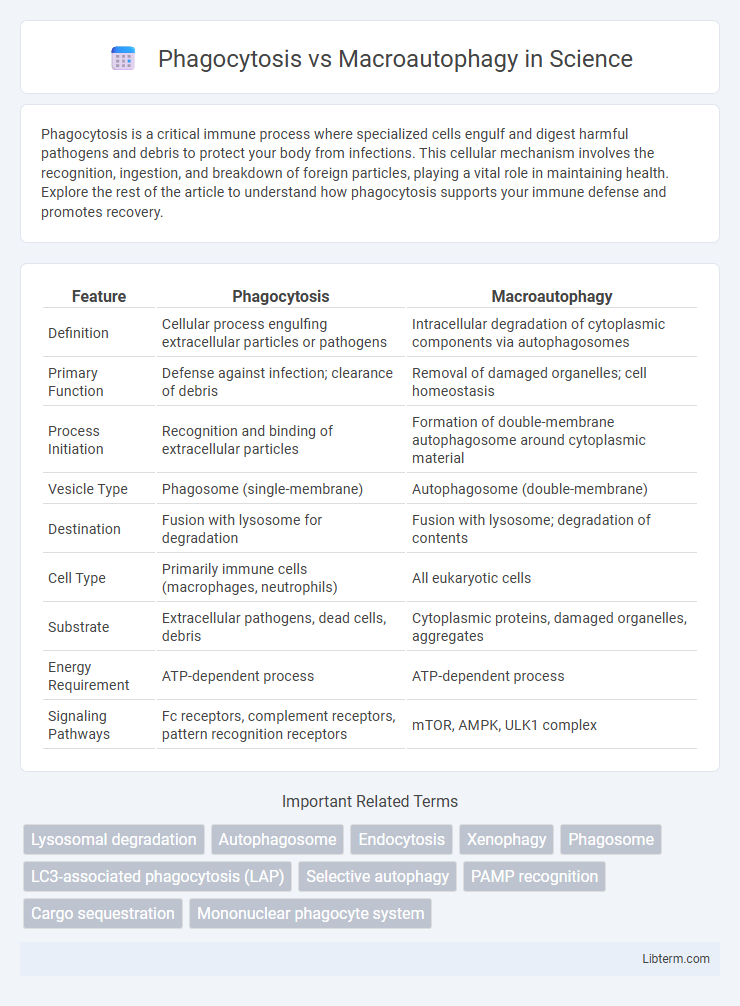
| Feature | Phagocytosis | Macroautophagy |
|---|---|---|
| Definition | Cellular process engulfing extracellular particles or pathogens | Intracellular degradation of cytoplasmic components via autophagosomes |
| Primary Function | Defense against infection; clearance of debris | Removal of damaged organelles; cell homeostasis |
| Process Initiation | Recognition and binding of extracellular particles | Formation of double-membrane autophagosome around cytoplasmic material |
| Vesicle Type | Phagosome (single-membrane) | Autophagosome (double-membrane) |
| Destination | Fusion with lysosome for degradation | Fusion with lysosome; degradation of contents |
| Cell Type | Primarily immune cells (macrophages, neutrophils) | All eukaryotic cells |
| Substrate | Extracellular pathogens, dead cells, debris | Cytoplasmic proteins, damaged organelles, aggregates |
| Energy Requirement | ATP-dependent process | ATP-dependent process |
| Signaling Pathways | Fc receptors, complement receptors, pattern recognition receptors | mTOR, AMPK, ULK1 complex |
Introduction to Phagocytosis and Macroautophagy
Phagocytosis is a cellular process where specialized cells engulf and digest extracellular particles, pathogens, or debris, playing a crucial role in immune defense and tissue homeostasis. Macroautophagy, often simply called autophagy, is a conserved intracellular degradation pathway that involves the sequestration of cytoplasmic components within double-membrane vesicles called autophagosomes, which then fuse with lysosomes for degradation and recycling. Both processes are essential for cellular maintenance but differ in their mechanisms and target substrates, with phagocytosis focusing on external material and macroautophagy targeting intracellular components.
Defining Cellular Degradation Pathways
Phagocytosis and macroautophagy are distinct cellular degradation pathways crucial for maintaining cellular homeostasis by removing unwanted materials. Phagocytosis is an extracellular process where cells engulf large particles or pathogens into phagosomes, which then fuse with lysosomes for degradation. In contrast, macroautophagy targets intracellular components, forming double-membraned autophagosomes that sequester cytoplasmic material and deliver it to lysosomes for recycling and degradation.
Key Mechanisms of Phagocytosis
Phagocytosis involves the recognition and binding of particles to cell surface receptors, triggering actin cytoskeleton remodeling that leads to membrane engulfment and formation of a phagosome. Key mechanisms include receptor-mediated signaling pathways such as Fc-gamma receptors and complement receptors, which initiate localized actin polymerization and pseudopod extension to surround targets. The subsequent phagosome maturation process involves fusion with lysosomes, forming phagolysosomes where enzymatic degradation of internalized material occurs.
Macroautophagy: Process and Regulation
Macroautophagy is a highly regulated cellular degradation pathway that involves the formation of double-membrane vesicles called autophagosomes, which engulf cytoplasmic components for lysosomal degradation. Key regulatory proteins such as ULK1, Beclin-1, and mTOR coordinate the initiation and progression of autophagosome formation in response to cellular stress and nutrient availability. This process plays a critical role in maintaining cellular homeostasis, removing damaged organelles, and recycling macromolecules to support cell survival under metabolic stress.
Origin of Vesicles: Phagosomes vs Autophagosomes
Phagosomes originate from the plasma membrane as it engulfs extracellular particles or pathogens through receptor-mediated endocytosis, forming a single-membrane vesicle enclosing the cargo. Autophagosomes arise from the isolation membrane, or phagophore, which is derived from intracellular organelles such as the endoplasmic reticulum, mitochondria, and Golgi apparatus, resulting in a double-membrane structure that sequesters cytoplasmic components. This key distinction in vesicle biogenesis underlies the different functional roles of phagocytosis in clearing external materials and macroautophagy in degrading intracellular components.
Molecular Players and Signaling Pathways
Phagocytosis involves membrane receptors such as Fc receptors and complement receptors, activating signaling cascades including PI3K and tyrosine kinases to facilitate engulfment of extracellular particles. Macroautophagy is regulated by the ULK1 complex, Beclin-1, and ATG proteins, orchestrated through mTOR and AMPK signaling pathways controlling autophagosome formation and lysosomal degradation. These distinct molecular players and pathways underscore the specific cellular mechanisms directing cargo recognition and processing during phagocytosis and macroautophagy.
Types of Cargo: External vs Internal Targets
Phagocytosis primarily engulfs external cargo such as bacteria, dead cells, and large particles, playing a crucial role in immune defense and tissue homeostasis. Macroautophagy targets internal cellular components like damaged organelles, protein aggregates, and invading intracellular pathogens, facilitating cellular quality control and nutrient recycling. The distinction in cargo origin--external for phagocytosis and internal for macroautophagy--highlights their complementary roles in maintaining cellular integrity and immune function.
Roles in Immunity and Homeostasis
Phagocytosis primarily functions as an immune defense mechanism by engulfing and degrading extracellular pathogens and apoptotic cells, thus maintaining tissue homeostasis and preventing infection. Macroautophagy targets intracellular components, including damaged organelles and protein aggregates, maintaining cellular homeostasis and supporting innate immunity by eliminating intracellular pathogens and modulating inflammation. Both processes are essential for immune regulation and cellular quality control, with phagocytosis specializing in extracellular clearance and macroautophagy ensuring intracellular degradation and metabolic balance.
Pathological Implications: Diseases and Dysfunctions
Phagocytosis dysfunction is closely linked to impaired immune responses, contributing to chronic infections and autoimmune disorders by failing to clear pathogens and apoptotic cells effectively. Macroautophagy defects are implicated in neurodegenerative diseases such as Parkinson's and Alzheimer's, where the accumulation of damaged proteins and organelles leads to cell death and tissue degeneration. Both processes, when dysregulated, play critical roles in cancer progression by either promoting tumor cell survival or impairing immune surveillance.
Comparative Summary: Phagocytosis vs Macroautophagy
Phagocytosis involves the engulfment of large extracellular particles or pathogens by specialized cells such as macrophages, whereas macroautophagy targets intracellular components like damaged organelles and protein aggregates for degradation. Phagocytosis relies on cell membrane extension to form a phagosome, which then fuses with lysosomes, while macroautophagy involves the formation of a double-membrane autophagosome that sequesters cytoplasmic content before lysosomal fusion. Both processes are critical for cellular homeostasis and immune defense but differ in mechanisms, triggers, and substrate specificity.
Phagocytosis Infographic
 libterm.com
libterm.com