Orthoclase and quartz are two common minerals found in many types of rocks, both playing a crucial role in Earth's crust composition. Orthoclase, a potassium feldspar, contributes to the formation of granite and other igneous rocks, while quartz, composed of silicon dioxide, is known for its hardness and resistance to weathering. Explore the article to understand their properties, uses, and significance in geology and everyday life.
Table of Comparison
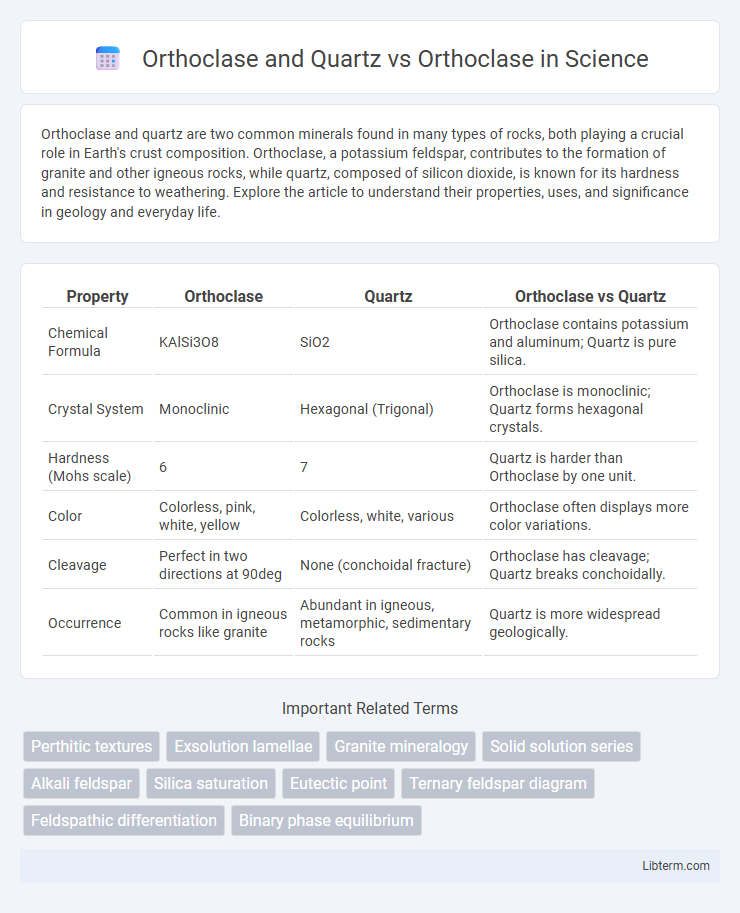
| Property | Orthoclase | Quartz | Orthoclase vs Quartz |
|---|---|---|---|
| Chemical Formula | KAlSi3O8 | SiO2 | Orthoclase contains potassium and aluminum; Quartz is pure silica. |
| Crystal System | Monoclinic | Hexagonal (Trigonal) | Orthoclase is monoclinic; Quartz forms hexagonal crystals. |
| Hardness (Mohs scale) | 6 | 7 | Quartz is harder than Orthoclase by one unit. |
| Color | Colorless, pink, white, yellow | Colorless, white, various | Orthoclase often displays more color variations. |
| Cleavage | Perfect in two directions at 90deg | None (conchoidal fracture) | Orthoclase has cleavage; Quartz breaks conchoidally. |
| Occurrence | Common in igneous rocks like granite | Abundant in igneous, metamorphic, sedimentary rocks | Quartz is more widespread geologically. |
Introduction to Orthoclase and Quartz
Orthoclase and Quartz are both essential minerals in the feldspar and silica groups, respectively, commonly found in igneous and metamorphic rocks. Orthoclase, a potassium-rich feldspar, plays a crucial role in granite formation, whereas Quartz, composed of silicon dioxide, is renowned for its hardness and chemical stability. Their distinct crystal structures and compositions make them fundamental components in geological studies and industrial applications alike.
Understanding the Composition of Orthoclase
Orthoclase, a potassium feldspar mineral with the chemical formula KAlSi3O8, primarily consists of potassium, aluminum, silicon, and oxygen, while quartz is composed solely of silicon dioxide (SiO2). Understanding orthoclase's composition highlights its role in igneous rocks, where it forms through the crystallization of magma and differs from quartz by containing aluminum and potassium. This compositional difference influences orthoclase's physical properties, such as its cleavage and hardness, distinguishing it from the more chemically pure and harder quartz.
Key Characteristics of Quartz
Quartz exhibits a crystalline structure composed of silicon dioxide (SiO2), characterized by its hardness of 7 on the Mohs scale, higher than orthoclase's 6. Its hexagonal crystal system contrasts with orthoclase's monoclinic structure, making quartz more resistant to weathering and erosion. Quartz's diverse color range and piezoelectric properties further distinguish it from orthoclase, which is primarily feldspar with potassium aluminum silicate composition.
Orthoclase vs Quartz: Chemical Structure
Orthoclase is a potassium feldspar with the chemical formula KAlSi3O8, characterized by a framework structure of interconnected silica (SiO4) and alumina (AlO4) tetrahedra, while quartz consists entirely of silicon dioxide (SiO2) with a continuous framework of SiO4 tetrahedra. The substitution of potassium ions in orthoclase creates differences in crystal lattice parameters and physical properties compared to pure quartz. This variation in chemical composition influences their stability, melting points, and occurrence in igneous and metamorphic rocks.
Physical Properties Comparison
Orthoclase exhibits a Mohs hardness of 6, while Quartz is harder with a rating of 7, making Quartz more resistant to scratching. Orthoclase has a specific gravity around 2.56 to 2.58, whereas Quartz has a slightly higher specific gravity of approximately 2.65. In terms of cleavage, Orthoclase shows good two-directional cleavage at 90 degrees, contrasting with Quartz's conchoidal fracture and lack of cleavage.
Geological Occurrence and Formation
Orthoclase and Quartz commonly coexist in igneous and metamorphic rocks, particularly granite and pegmatite, where Orthoclase forms through slow cooling processes allowing large crystal growth, while Quartz crystallizes from silica-rich fluids. Orthoclase typically crystallizes at temperatures between 650-750degC in felsic magma environments, whereas Quartz forms at a wider range of temperatures due to its pure silica composition. The distinct crystallization conditions and chemical compositions of Orthoclase and Quartz influence their geological occurrence, with Orthoclase often indicating high-temperature magmatic origins and Quartz signifying variable temperature conditions in both magmatic and hydrothermal systems.
Industrial and Commercial Uses
Orthoclase and quartz, both feldspar minerals, have distinct industrial and commercial applications due to their differing physical properties. Orthoclase is primarily used in ceramics and glass manufacturing because of its high potassium content, which improves durability and color quality, while quartz, valued for its hardness and chemical stability, is essential in producing abrasives, optical instruments, and silicon chips. Compared to orthoclase alone, combining quartz with orthoclase enhances product strength and precision in electronics and construction materials.
Optical and Aesthetic Differences
Orthoclase and Quartz differ significantly in optical properties; Orthoclase exhibits lower birefringence and a distinct cleavage pattern, whereas Quartz shows higher birefringence with no cleavage, resulting in differing light refraction and internal reflections. Aesthetically, Orthoclase often presents a pearly luster and pastel shades like pink or cream, contrasting with Quartz's glassy luster and broader color range, from clear to smoky or rose hues. These differences influence their use in jewelry and industrial applications, with Quartz preferred for clarity and brilliance, while Orthoclase is valued for its unique texture and subtler shimmer.
Identification and Distinguishing Features
Orthoclase and quartz both belong to the silicate mineral family but differ significantly in identification and distinguishing features. Orthoclase is a potassium feldspar characterized by its monoclinic crystal system, hardness of 6 on the Mohs scale, and often shows cleavage planes at nearly right angles, whereas quartz has a hexagonal crystal system, a higher hardness of 7, and exhibits conchoidal fracture without cleavage. In terms of color and luster, orthoclase typically appears white, pink, or flesh-colored with a vitreous to pearly luster, while quartz ranges widely in color and features a glassy, vitreous luster.
Summary: Orthoclase and Quartz vs Orthoclase
Orthoclase and Quartz are both essential minerals in the feldspar and silica groups, respectively, with Orthoclase being a potassium-rich feldspar and Quartz composed of silicon dioxide. Compared to Orthoclase alone, the combination of Orthoclase and Quartz enhances rock durability and resistance to weathering due to Quartz's hardness and chemical stability. This mineral pairing frequently occurs in granite, influencing its texture and structural properties essential for construction and geological studies.
Orthoclase and Quartz Infographic
 libterm.com
libterm.com