Dilute refers to the process of reducing the concentration of a substance in a solution, often by adding more solvent such as water. This technique is essential in chemistry, cooking, and medicine to achieve the desired strength or consistency. Explore the article to learn how dilution impacts various applications and why it matters to your daily life.
Table of Comparison
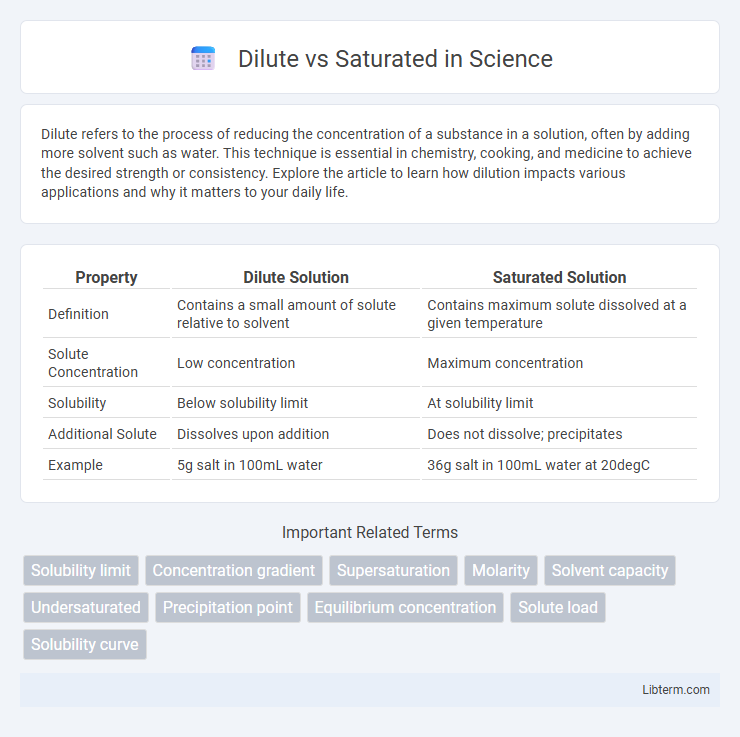
| Property | Dilute Solution | Saturated Solution |
|---|---|---|
| Definition | Contains a small amount of solute relative to solvent | Contains maximum solute dissolved at a given temperature |
| Solute Concentration | Low concentration | Maximum concentration |
| Solubility | Below solubility limit | At solubility limit |
| Additional Solute | Dissolves upon addition | Does not dissolve; precipitates |
| Example | 5g salt in 100mL water | 36g salt in 100mL water at 20degC |
Understanding Dilute and Saturated Solutions
Dilute solutions contain a relatively small amount of solute compared to the solvent, resulting in lower concentration levels, whereas saturated solutions hold the maximum amount of solute that can dissolve at a given temperature and pressure. Understanding the difference between dilute and saturated solutions is key for predicting solubility, chemical reactions, and concentration changes in various scientific and industrial processes. The saturation point indicates equilibrium between dissolved and undissolved solute, while dilution refers to reducing solute concentration by increasing solvent volume.
Key Differences Between Dilute and Saturated Solutions
Dilute solutions contain a relatively small amount of solute compared to the solvent, resulting in low concentration, while saturated solutions have the maximum amount of solute dissolved at a given temperature and pressure, with no more solute able to dissolve. The key difference lies in solubility; dilute solutions are unsaturated and can dissolve additional solute, whereas saturated solutions reach equilibrium and any added solute remains undissolved. Concentration gradients and solute-solvent interactions significantly influence the physical properties and dissolving capacity distinguishing dilute from saturated solutions.
Characteristics of Dilute Solutions
Dilute solutions contain a relatively low concentration of solute compared to the solvent, resulting in weaker intermolecular forces and lower boiling points than saturated solutions. They exhibit greater solvent dominance, allowing for higher solubility capacity and easier solute dissolution. The low solute concentration in dilute solutions also means minimal impact on properties like osmotic pressure and vapor pressure.
Characteristics of Saturated Solutions
Saturated solutions contain the maximum amount of solute that can dissolve at a given temperature, resulting in a dynamic equilibrium between dissolved and undissolved solute. Characteristics of saturated solutions include the presence of excess solute that no longer dissolves, constant concentration despite added solute, and temperature-dependent solubility limits. These solutions are often used to study solubility curves and predict crystallization or precipitation phenomena.
Factors Affecting Solution Concentration
Solution concentration depends on factors such as the amount of solute dissolved, the volume of solvent present, and temperature, which influences solubility rates. Dilute solutions contain a smaller concentration of solute relative to solvent, while saturated solutions hold the maximum solute possible at a given temperature. Pressure changes primarily affect gas solubility, altering saturation levels in solutions containing gaseous solutes.
Examples of Dilute and Saturated Solutions
A dilute solution contains a small amount of solute relative to the solvent, such as a teaspoon of salt dissolved in a liter of water, which results in a weak flavor or low concentration. In contrast, a saturated solution holds the maximum amount of solute that can dissolve at a given temperature, like sugar added to water until no more dissolves, forming sugar crystals at the bottom. Examples of dilute solutions include lightly salted water and weak tea, while examples of saturated solutions include seawater at its salt limit and saturated sugar syrup used in baking.
Methods to Identify Saturated and Dilute Solutions
Methods to identify saturated and dilute solutions include observing solubility limits, where a saturated solution contains the maximum solute dissolved at a given temperature, often evidenced by undissolved solute at the bottom of the container. Conducting a saturation test by adding small increments of solute and noting whether it dissolves or precipitates helps differentiate between dilute and saturated solutions. Measuring concentration through tools such as a refractometer, conductivity meter, or titration also provides quantitative identification of solution saturation levels.
Importance in Chemical Reactions
Dilute solutions have a lower concentration of solute, which slows reaction rates by reducing the frequency of effective molecular collisions, making them essential for controlling reaction speed and selectivity. Saturated solutions contain the maximum amount of solute dissolved at a given temperature, often reaching equilibrium where no more solute dissolves, influencing precipitation and crystallization processes in chemical reactions. Understanding the distinction between dilute and saturated solutions is crucial for optimizing reaction conditions, yield, and product purity in industrial and laboratory chemical processes.
Practical Applications in Daily Life
Dilute solutions, such as weak tea or saline eye drops, provide mild concentrations suitable for everyday consumption and gentle medical applications. Saturated solutions appear in processes like making rock candy, where sugar is dissolved to its maximum capacity, or in saltwater aquariums requiring stable salinity levels. Understanding the difference between dilute and saturated solutions aids in cooking, pharmaceuticals, and environmental science, optimizing outcomes and safety in daily tasks.
Summary: Choosing Between Dilute and Saturated Solutions
Choosing between dilute and saturated solutions depends on the desired concentration for a specific application. Dilute solutions contain a lower concentration of solute, providing mild reactivity and easier handling, while saturated solutions hold the maximum solute amount, offering stronger effects and stability. Understanding the solubility limits and purpose ensures optimal performance in chemical reactions, industrial processes, or laboratory experiments.
Dilute Infographic
 libterm.com
libterm.com