Gamma rays are high-energy electromagnetic waves produced by nuclear reactions, cosmic phenomena, and radioactive decay. These rays penetrate materials deeply, making them crucial in medical treatments like cancer radiotherapy and in scientific research for imaging and spectroscopy. Discover how gamma rays impact technology and health in the rest of this article.
Table of Comparison
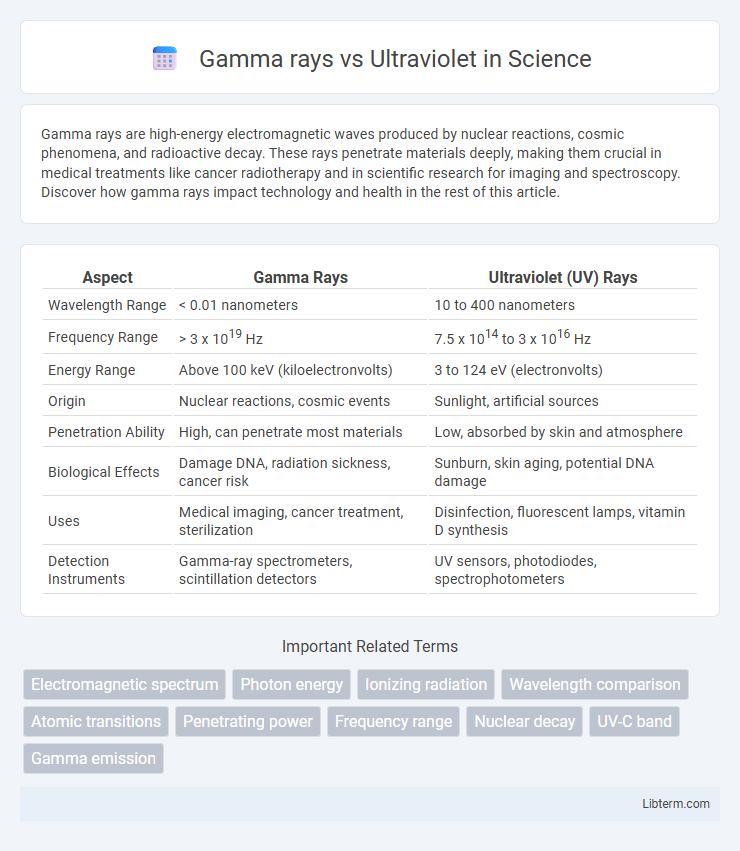
| Aspect | Gamma Rays | Ultraviolet (UV) Rays |
|---|---|---|
| Wavelength Range | < 0.01 nanometers | 10 to 400 nanometers |
| Frequency Range | > 3 x 1019 Hz | 7.5 x 1014 to 3 x 1016 Hz |
| Energy Range | Above 100 keV (kiloelectronvolts) | 3 to 124 eV (electronvolts) |
| Origin | Nuclear reactions, cosmic events | Sunlight, artificial sources |
| Penetration Ability | High, can penetrate most materials | Low, absorbed by skin and atmosphere |
| Biological Effects | Damage DNA, radiation sickness, cancer risk | Sunburn, skin aging, potential DNA damage |
| Uses | Medical imaging, cancer treatment, sterilization | Disinfection, fluorescent lamps, vitamin D synthesis |
| Detection Instruments | Gamma-ray spectrometers, scintillation detectors | UV sensors, photodiodes, spectrophotometers |
Introduction to Gamma Rays and Ultraviolet Radiation
Gamma rays are high-energy electromagnetic waves with frequencies above 10^19 Hz, originating from nuclear reactions and cosmic phenomena, exhibiting extreme penetrating power useful in medical imaging and cancer treatment. Ultraviolet (UV) radiation spans frequencies from 7.5 x 10^14 to 3 x 10^16 Hz and is primarily emitted by the sun, playing a crucial role in vitamin D synthesis but posing risks like skin damage and DNA mutation. Both gamma rays and UV radiation lie beyond the visible light spectrum, distinguished by their energy levels, sources, and biological effects.
Understanding the Electromagnetic Spectrum
Gamma rays have the shortest wavelengths and highest frequencies in the electromagnetic spectrum, enabling them to penetrate most materials and ionize atoms, which is crucial in medical and scientific applications. Ultraviolet rays occupy a higher frequency range than visible light but lower than gamma rays, playing a significant role in processes such as vitamin D synthesis and sterilization. Understanding the energy levels, wavelengths, and frequencies of these electromagnetic waves is essential for harnessing their unique properties across various technological and environmental fields.
Key Differences Between Gamma Rays and Ultraviolet
Gamma rays have significantly higher frequencies and shorter wavelengths than ultraviolet rays, resulting in greater energy and deeper penetration capabilities. While gamma rays originate from nuclear reactions or cosmic events, ultraviolet rays primarily come from the sun and artificial sources like black lights. The distinct energy levels cause gamma rays to ionize atoms and damage DNA more severely, making them more hazardous compared to ultraviolet radiation.
Sources of Gamma Rays vs. Ultraviolet Light
Gamma rays originate from high-energy processes such as nuclear reactions, cosmic phenomena like supernovae, and radioactive decay, producing photons with the highest energy in the electromagnetic spectrum. Ultraviolet light primarily comes from the Sun's surface and artificial sources like mercury-vapor lamps and black lights, with photon energies lower than gamma rays but higher than visible light. The distinct sources reflect differences in energy production mechanisms, with gamma rays tied to atomic-scale processes and ultraviolet light linked to electronic transitions in atoms.
Penetrating Power: Gamma Rays vs. Ultraviolet
Gamma rays exhibit significantly higher penetrating power than ultraviolet rays, capable of passing through most materials, including human tissue and dense metals, due to their extremely short wavelengths and high energy photons. Ultraviolet rays have limited penetration, primarily absorbed by the outer layers of the skin or thin materials, restricting their impact to surface-level interactions. This distinction in penetrating ability makes gamma rays critical in medical imaging and cancer treatments, while ultraviolet rays are mainly effective for sterilization and surface-level applications.
Biological Effects and Health Risks
Gamma rays, with their extremely high energy and deep tissue penetration, pose significant biological risks including DNA damage, increased cancer risk, and acute radiation sickness. Ultraviolet (UV) radiation primarily affects skin and eyes, causing sunburn, skin aging, and a higher likelihood of skin cancers such as melanoma. Prolonged exposure to gamma rays leads to systemic health issues, while UV exposure mainly results in localized damage with long-term effects on skin health.
Applications in Medicine and Technology
Gamma rays are utilized in cancer treatment through targeted radiotherapy, leveraging their high energy to destroy malignant cells with precision. Ultraviolet light plays a crucial role in sterilization and disinfection processes within medical settings due to its ability to inactivate bacteria and viruses. Both electromagnetic waves contribute significantly to advanced imaging technologies; gamma rays enable precise diagnostic imaging in nuclear medicine, while ultraviolet radiation aids in phototherapy for skin conditions and forensic analysis.
Detection and Measurement Techniques
Gamma rays are detected using scintillation detectors, semiconductor detectors, and gas-filled detectors, which measure the high-energy photons through ionization and light emission processes. Ultraviolet light detection relies on photodiodes, photomultiplier tubes, and charge-coupled devices (CCDs) that measure photon energy in the UV spectrum via photoelectric or photoemission effects. Measurement techniques for gamma rays emphasize precise energy resolution and high penetration detection, while UV measurement focuses on spectral sensitivity and intensity calibration within specific wavelength bands.
Safety Precautions and Exposure Limits
Gamma rays, characterized by extremely high energy and penetrating power, require stringent safety precautions such as lead shielding and remote handling to minimize exposure, with regulatory limits typically set at 0.001 mSv per year for the general public. Ultraviolet (UV) radiation exposure, primarily from UV-A, UV-B, and UV-C rays, mandates protective measures including sunscreen, UV-blocking eyewear, and limiting direct sun exposure to prevent skin damage and eye injuries; occupational exposure limits are generally set at 30 J/m2 for UV-C and vary for UV-A and UV-B based on wavelength and intensity. Both gamma rays and ultraviolet radiation demand controlled environments and adherence to exposure guidelines from agencies like the International Commission on Radiological Protection (ICRP) and the American Conference of Governmental Industrial Hygienists (ACGIH) to ensure safety.
Summary: Gamma Rays vs. Ultraviolet Radiation
Gamma rays possess much higher energy and shorter wavelengths compared to ultraviolet radiation, enabling them to penetrate materials more deeply and cause more significant ionization effects. Ultraviolet rays, with wavelengths ranging from 10 nm to 400 nm, are less energetic and primarily affect the surface of materials, contributing to skin tanning and vitamin D synthesis. Gamma rays, emitted by radioactive decay and cosmic phenomena, are utilized in medical imaging and cancer treatment, while ultraviolet radiation is commonly involved in sterilization and fluorescent lighting.
Gamma rays Infographic
 libterm.com
libterm.com