Luminescence is the emission of light by a substance that has not been heated, often resulting from chemical reactions, electrical energy, or biological processes. This fascinating phenomenon enables applications ranging from glow-in-the-dark materials to advanced medical imaging techniques. Explore the rest of this article to uncover how luminescence impacts science and everyday life.
Table of Comparison
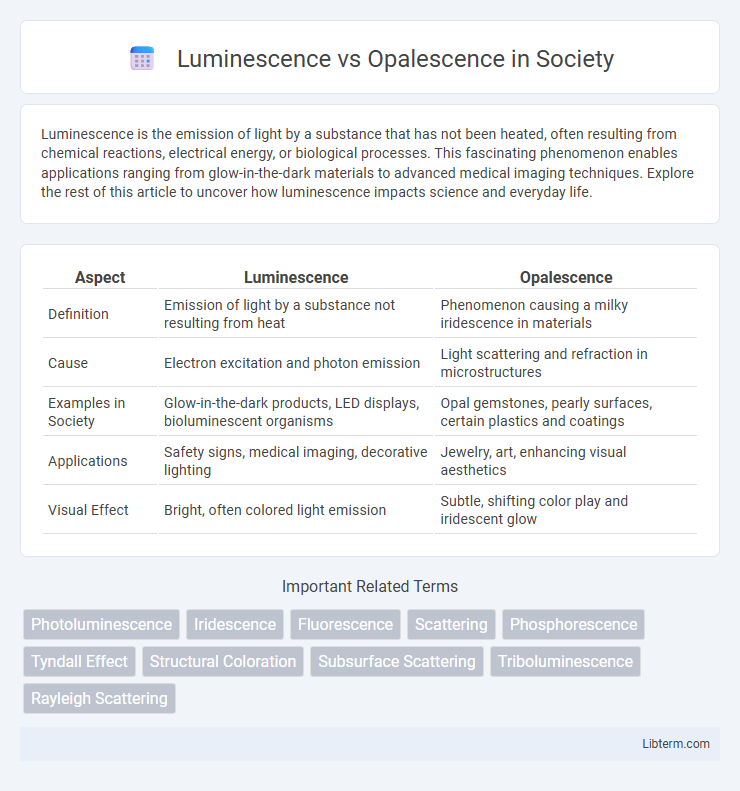
| Aspect | Luminescence | Opalescence |
|---|---|---|
| Definition | Emission of light by a substance not resulting from heat | Phenomenon causing a milky iridescence in materials |
| Cause | Electron excitation and photon emission | Light scattering and refraction in microstructures |
| Examples in Society | Glow-in-the-dark products, LED displays, bioluminescent organisms | Opal gemstones, pearly surfaces, certain plastics and coatings |
| Applications | Safety signs, medical imaging, decorative lighting | Jewelry, art, enhancing visual aesthetics |
| Visual Effect | Bright, often colored light emission | Subtle, shifting color play and iridescent glow |
Understanding Luminescence: Definition and Principles
Luminescence is the emission of light by a substance that has absorbed energy, occurring without significant heat generation, and it includes phenomena such as fluorescence and phosphorescence. This process involves the excitation of electrons to higher energy states, followed by the release of photons as they return to their ground state, which distinguishes it from thermal radiation. Understanding the principles of luminescence is crucial for applications in fields like material science, bioimaging, and forensic analysis, where light emission properties are harnessed for detection and visualization.
What is Opalescence? Key Characteristics
Opalescence is the optical phenomenon where a material displays a play of colors with a milky or pearly appearance caused by light scattering within microstructures. Key characteristics include a translucent, milky base color with shifting hues, often blue, white, or rainbow-like, depending on the viewing angle and lighting. This effect is distinct from luminescence, which involves emission of light by a substance not caused by heat.
The Science Behind Luminescence
Luminescence is the emission of light by a substance not resulting from heat, caused by electronic transitions within molecules or atoms that release photons when excited by energy sources such as UV light. This phenomenon differs from opalescence, which is caused by the scattering of light in a material with a heterogeneous structure, leading to a milky or iridescent appearance. Understanding luminescence involves studying photoluminescence mechanisms, including fluorescence and phosphorescence, which depend on the molecular or atomic energy states and their ability to absorb and emit light efficiently.
How Opalescence Occurs: Mechanisms Explained
Opalescence occurs due to the diffraction and scattering of light by microscopic silica spheres arranged in a regular pattern within a material, creating its characteristic milky and iridescent appearance. Unlike luminescence, which involves the emission of light from excited electrons returning to a ground state, opalescence is a physical optical effect caused by the interaction of light with nanostructured surfaces. The specific size and arrangement of these silica spheres determine the colors and intensity observed in opalescent substances.
Visual Differences: Luminescence vs Opalescence
Luminescence refers to the emission of light by a substance that has absorbed energy, producing a glowing effect often visible in low light conditions, whereas opalescence is characterized by a milky, iridescent appearance caused by light scattering within microstructures, creating shimmering colors. Visually, luminescent materials emit light in a specific wavelength that can appear as a distinct glow, while opalescent materials display subtle color shifts and a pearly sheen without emitting actual light. The key difference lies in luminescence being an active light emission phenomenon, compared to opalescence, which is a passive optical effect based on light diffraction and scattering.
Natural Examples of Luminescence
Natural luminescence occurs in various organisms such as fireflies, which produce light through bioluminescence for communication and mating purposes. Deep-sea creatures like the anglerfish emit light to attract prey and navigate dark ocean environments, demonstrating chemiluminescent properties. Unlike opalescence, which is a visual phenomenon involving light diffraction in materials like opals, luminescence involves the emission of light from natural sources without heat.
Opalescence in Nature and Everyday Life
Opalescence in nature is commonly observed in gemstones like opals, where the diffraction of light causes a shifting play of colors known as "play-of-color," enhancing their iridescent beauty. Everyday objects such as soap bubbles, certain minerals, and even the feathers of some birds exhibit opalescence through the scattering and interference of light within microscopic structures. Unlike luminescence, which involves the emission of light through chemical or biological processes, opalescence is purely optical, resulting from light interaction with complex surfaces or materials.
Applications: Uses of Luminescence in Technology
Luminescence finds extensive applications in technology, including organic light-emitting diodes (OLEDs) for advanced display screens and efficient lighting solutions. It is also pivotal in bioimaging and medical diagnostics, enabling non-invasive visualization of cellular processes using fluorescent markers. Moreover, luminescent materials are used in security features for anti-counterfeiting measures and in optoelectronic devices such as lasers and sensors.
Opalescence in Art, Jewelry, and Design
Opalescence in art, jewelry, and design emphasizes the captivating play of colors and milky translucence that mimic the natural qualities of opal gemstones, creating dynamic visual effects that enhance aesthetic appeal. This phenomenon is utilized to infuse depth and iridescence into materials, from precious stones to glass and ceramics, making pieces appear vibrant and alive under varying light conditions. Unlike luminescence, which involves emitted light, opalescence reflects and scatters light, giving objects a unique and mesmerizing glow highly prized in luxury design and fine craftsmanship.
Comparing Luminescence and Opalescence: Key Takeaways
Luminescence refers to the emission of light by a substance not resulting from heat, usually producing a glow in various colors under ultraviolet or visible light, while opalescence is the phenomenon of materials displaying a milky or pearly iridescence, often shifting colors based on the angle of light. Key differences include luminescence being an active light emission process and opalescence being a passive light scattering effect, primarily observed in materials like opal gemstones or certain plastics. Understanding these distinctions is crucial in fields such as gemology, material science, and optical applications for correctly identifying and utilizing these optical phenomena.
Luminescence Infographic
 libterm.com
libterm.com